 W
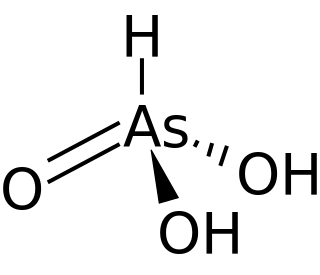
WArsonic acid is the simplest of the arsonic acids. It is a hypothetical compound, although the tautomeric arsenious acid (As(OH)3) is well established. In contrast to the instability of HAsO(OH)2, the phosphorus compound with analogous stoichiometry exists as the tetrahedral tautomer. Similarly, organic derivatives such as phenylarsonic acid are tetrahedral with pentavalent central atom.
 W
WBenzotriyne or cyclo[6]carbon is a hypothetical chemical compound, an allotrope of carbon with molecular formula C6. The molecule is a ring of six carbon atoms, connected by alternating triple and single bonds. It is, therefore, a potential member of the cyclo[n]carbon family.
 W
WBismole is a theoretical heterocyclic organic compound, a five-membered ring with the formula C4H4BiH. It is classified as a metallole. It can be viewed as a structural analog of pyrrole, with bismuth replacing the nitrogen atom of pyrrole. The unsubstituted compound has not been isolated due to the high energy of the Bi-H bond. Substituted derivatives, which have been synthesized, are called bismoles.
 W
WBorabenzene is a hypothetical organoboron compound with the formula C5H5B. Unlike the related but highly stable benzene molecule, borabenzene would be electron-deficient. Related derivatives are the boratabenzene anions, including the parent [C5H5BH]−.
 W
WBromochlorofluoroiodomethane is a hypothetical haloalkane with all four stable halogen substituents present in it.
 W
WCalicene or triapentafulvalene is a hydrocarbon of the fulvalene class with chemical formula C8H6, composed of a cyclopentadiene ring and a cyclopropene ring linked by a double bond. Its name is derived from the Latin calix meaning "goblet", from its shape.
 W
WClaus' benzene (C6H6) is a hypothetical hydrocarbon and an isomer of benzene. It was proposed by Adolf Karl Ludwig Claus in 1867 as a possible structure for benzene at a time when the structure of benzene was still being debated. The molecule can be described as a hexagon with carbon atoms positioned at the corners, with each carbon connected to its two ortho carbons (the nearest carbons) and the one para carbon connected diametrically. High strain energy makes its synthesis impossible. Although it is often referred to alongside Dewar benzene and prismane, it is not possible to synthesise it, while Dewar benzene and prismane can be.
 W
WCyclobutanetetrone, also called tetraoxocyclobutane, is an organic compound with formula C4O4 or (CO)4, the fourfold ketone of cyclobutane. It would be an oxide of carbon, indeed a tetramer of carbon monoxide.
 W
WBenzotriyne or cyclo[6]carbon is a hypothetical chemical compound, an allotrope of carbon with molecular formula C6. The molecule is a ring of six carbon atoms, connected by alternating triple and single bonds. It is, therefore, a potential member of the cyclo[n]carbon family.
 W
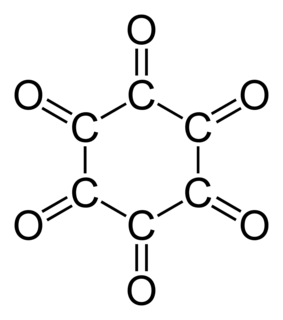
WCyclohexanehexone, also known as hexaketocyclohexane and triquinoyl, is an organic compound with formula C6O6, the sixfold ketone of cyclohexane. It is an oxide of carbon (an oxocarbon), a hexamer of carbon monoxide.
 W
WCyclopentanepentone, also known as leuconic acid, is a hypothetical organic compound with formula C5O5, the fivefold ketone of cyclopentane. It would be an oxide of carbon (an oxocarbon), indeed a pentamer of carbon monoxide.
 W
WCyclopropatriene is a hypothetical allotrope of carbon that was once proposed as a candidate for a spectroscopically observed tricarbon species. It is a cyclic cumulene.
 W
WDichlorine pentoxide is a hypothetical chlorine oxide with a chemical formula Cl2O5. It remains unknown. Theory predicts that the perchloryl/chloride peroxide structure would be the most stable among various isomers of this molecular formula, such as the anhydride of chloric acid or the chlorous acid/perchloric acid mixed anhydride.
 W
WDihydroimidazol-2-ylidene is a hypothetical organic compound with formula C3H6N2. It would be a heterocyclic compound, formally derived from imidazolidine with two hydrogen atoms removed from carbon number 2, leaving two vacant chemical bonds — which makes it a carbene.
 W
WDimethanospiro[2.2]octaplane is a hypothetical saturated hydrocarbon that is expected to have a carbon atom in with a stable, unusual square-planar coordination rather than the usual tetrahedral geometry of a carbon atom with four bonds.
 W
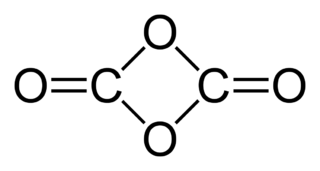
WThe chemical compound 1,3-dioxetanedione, or 1,3-dioxacyclobutane-2,4-dione is a hypothetical oxide of carbon with formula C2O4. It can be considered a cyclic dimer of carbon dioxide (CO2) or as a double ketone of 1,3-dioxetane (1,3-dioxacyclobutane).
 W
W1,2-Dioxin is a heterocyclic, organic, antiaromatic compound with the chemical formula C4H4O2. It is an isomeric form of 1,4-dioxin (or p-dioxin).
 W
WDisulfurous acid or pyrosulfurous acid is an oxoacid of sulfur with the formula H2S2O5. The salts of disulfurous acid are called disulfites or metabisulfites. Disulfurous acid is, like sulfurous acid (H2SO3), a phantom acid, which does not exist in the free state. In contrast to disulfate (S2O−7), disulfite has two directly connected sulfur atoms. The oxidation state of the sulfur atom bonded to three oxygen atoms is +5 while that of the other is +3.
 W
WEthylene dione or ethylenedione, also called dicarbon dioxide, ethenedione, or ethene-1,2-dione, is a chemical compound with the formula C2O2 or O=C=C=O. It is an oxide of carbon, and can be described as the carbon-carbon covalent dimer of carbon monoxide. It can also be thought of as the dehydrated form of glyoxylic acid, or a ketone of ethenone H2C=C=O.
 W
WFrancium hydroxide is a hypothetical inorganic compound with a chemical formula FrOH. It is francium's hydroxide.
 W
WGold iodide (AuI3) is the chemical compound with the formula AuI3. Although Au2I6 is predicted to be stable, gold(III) iodide remains an example of a nonexistent or unstable compound. Attempts to isolate pure samples result in the formation of gold(I) iodide and iodine:AuI3 → AuI + I2
 W
WHexaphenylethane is a hypothetical organic compound consisting of an ethane core with six phenyl substituents. All attempts at its synthesis have been unsuccessful. The trityl free radical, Ph3C ·, was originally thought to dimerize to form hexaphenylethane. However, an inspection of the NMR spectrum of this dimer reveals that it is in fact a non-symmetrical species, Gomberg's dimer instead.
 W
WHexaphosphabenzene is a valence isoelectronic analogue of benzene and is expected to have a similar planar structure due to resonance stabilization. Although several other allotropes of phosphorus are stable, no evidence for the existence of P6 has been reported. Preliminary ab initio calculations on the trimerisation of P2 leading to the formation of the cyclic P6 were performed, and it was predicted that hexaphosphabenzene would decompose to free P2 with an energy barrier of 13−15.4 kcal mol−1, and would therefore not be observed in the uncomplexed state under normal experimental conditions. The presence of an added solvent, such as ethanol, might lead to the formation of intermolecular hydrogen bonds which may block the destabilizing interaction between phosphorus lone pairs and consequently stabilize P6. The moderate barrier suggests that hexaphosphabenzene could be synthesized from a [2+2+2] cycloaddition of three P2 molecules. Currently, this is a synthetic endeavour which remains to be conquered.
 W
WHexazine is a hypothetical allotrope of nitrogen composed of 6 nitrogen atoms arranged in a ring-like structure analogous to that of benzene. It would be the final member of the azabenzene (azine) series, in which all of the methine groups of the benzene molecule have been replaced with nitrogen atoms. The two last members of this series, hexazine and pentazine, have not been observed, although all other members of the azine series have.
 W
WHypercubane is a hypothetical polycyclic hydrocarbon with the chemical formula C40H24. It is a molecular analog of the four-dimensional hypercube or tesseract. Hypercubane possesses an unconventional geometry of the carbon framework. It has Oh symmetry like classic cubane C8H8. The structure is that of octamethyl cubane—a carbon attached to each corner of cubane itself—having each of those carbon substituents joined to each of its neighbors by an ethylene-1,2-diyl linker to form an outer cage. The edge of each inner core and its outer linker form a cyclohexene.
 W
WMercury(IV) fluoride, HgF4, is the first mercury compound to be reported with mercury in the +4 oxidation state. Mercury, like the other group 12 elements (cadmium and zinc), has an s2d10 electron configuration and generally only forms bonds involving its 6s orbital. This means that the highest oxidation state mercury normally attains is +2, and for this reason it is usually considered a post-transition metal instead of a transition metal. HgF4 was first reported from experiments in 2007, but its existence remains disputed; experiments conducted in 2008 could not replicate the compound.
 W
WThe ammonium cation is a positively charged polyatomic ion with the chemical formula NH+4. It is formed by the protonation of ammonia. Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic groups.
 W
WOctaazacubane is a hypothetical explosive allotrope of nitrogen with formula N8, whose molecules have eight atoms arranged into a cube. (By comparison, nitrogen usually occurs as the diatomic molecule N2.) It can be regarded as a cubane-type cluster, where all eight corners are nitrogen atoms bonded along the edges. It is predicted to be a metastable molecule, in which despite the thermodynamic instability caused by bond strain, and the high energy of the N–N single bonds, the molecule remains kinetically stable for reasons of orbital symmetry.
 W
WA Platonic hydrocarbon is a hydrocarbon (molecule) whose structure matches one of the five Platonic solids, with carbon atoms replacing its vertices, carbon–carbon bonds replacing its edges, and hydrogen atoms as needed.
 W
WOrthoacetic acid or ethane-1,1,1-triol is an hypothetical organic compound with formula C2H6O3 or H3C-C(OH)3. It would be an ortho acid with the ethane backbone.
 W
WOrthocarbonic acid (methanetetrol) is the name given to a hypothetical compound with the chemical formula H4CO4 or C(OH)4. Its molecular structure consists of a single carbon atom bonded to four hydroxy groups. It would be therefore a fourfold alcohol. In theory it could lose four protons to give the hypothetical oxocarbon anion CO4−4 (orthocarbonate), and is therefore considered an oxoacid of carbon.
 W
WOrthoformic acid or methanetriol is a hypothetical compound with the formula HC(OH)3. In this molecule, the central carbon atom is bound to one hydrogen and three hydroxyl groups.
 W
WOxalic anhydride or ethanedioic anhydride, also called oxiranedione, is a hypothetical organic compound, one of several isomers having the formula C2O3 that have been studied computationally. It can be viewed as the anhydride of oxalic acid or the two-fold ketone of ethylene oxide. It is an oxide of carbon (an oxocarbon).
 W
WOxirene is a hypothesized heterocyclic chemical compound which contains an unsaturated three-membered ring containing two carbon atoms and one oxygen atom. Because it has never been observed, the substance is mainly studied by quantum chemical computational techniques. As the configuration is extremely strained and proposed to be an anti-aromatic 4 π electron system, oxirene is expected to be very high energy and unstable according to these calculations. Moreover, different computational methods draw different conclusions as to whether the structure constitutes a true molecule or merely a transition state between two isomeric molecular species.
 W
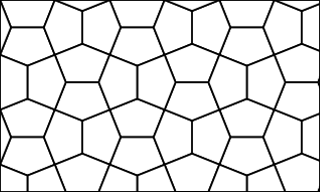
WPenta-graphene is a hypothetical carbon allotrope composed entirely of carbon pentagons and resembling the Cairo pentagonal tiling. Penta-graphene was proposed in 2014 on the basis of analyses and simulations. Further calculations showed that it is unstable in its pure form, but can be stabilized by hydrogenation. Owing to its atomic configuration, penta-graphene has an unusually negative Poisson’s ratio and very high ideal strength believed to exceed that of a similar material, graphene.
 W
WA Platonic hydrocarbon is a hydrocarbon (molecule) whose structure matches one of the five Platonic solids, with carbon atoms replacing its vertices, carbon–carbon bonds replacing its edges, and hydrogen atoms as needed.
 W
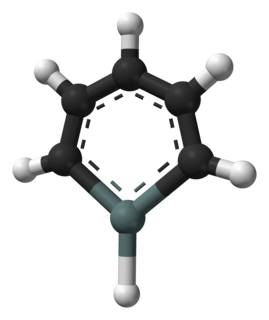
WStannabenzene (C5H6Sn) is the parent representative of a group of organotin compounds that are related to benzene with a carbon atom replaced by a tin atom. Stannabenzene itself has been studied by computational chemistry, but has not been isolated.
 W
WStibole is a theoretical heterocyclic organic compound, a five-membered ring with the formula C4H4SbH. It is classified as a metallole. It can be viewed as a structural analog of pyrrole, with antimony replacing the nitrogen atom of pyrrole. Substituted derivatives, which have been synthesized, are called stiboles.
 W
WTetra-tert-butylethylene is a hypothetical organic compound, a hydrocarbon with formula C18H36, or ((H3C−)3C−)2C=C(−C(−CH3)3)2. As the name indicates, its molecular structure can be viewed as an ethylene molecule H2C=CH2 with the four hydrogens replaced by tert-butyl −C(−CH3)3 groups.
 W
WTetraaminoethylene is a hypothetical organic compound with formula C2N4H8 or (H2N)2C=C(NH2)2. Like all geminal polyamines, this compound has never been synthesised and is believed to be extremely unstable.
 W
WTetrahedrane is a hypothetical platonic hydrocarbon with chemical formula C4H4 and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized as of 2021. However, a number of derivatives have been prepared. In a more general sense, the term tetrahedranes is used to describe a class of molecules and ions with related structure, e.g. white phosphorus.
 W
WTetranitratoxycarbon, systematic name tetra(nitrato-O,O,O-methyl)methane, is a hypothetical molecule that was proposed by Clara Lazen, a fifth-grader in Kansas City, Missouri, who conceived of its structure and built a model in 2012. She is credited as co-author of a scientific paper on the molecule, which uses computational chemistry to predict that the molecule could actually exist.
 W
WTriafulvalene or cyclopropenylidenecyclopropene is a fulvalene hydrocarbon with chemical formula C6H4, composed of two linked cyclopropene rings. Triafulvalene has never been isolated, since it can decompose via an isodesmic reaction. However, this molecule is of theoretical significance for theoretical organic chemists, and its structure, stability, and spectral properties are well-studied.
 W
WThe triboracyclopropenyl fragment is a cyclic structural motif in boron chemistry, named for its geometric similarity to cyclopropene. In contrast to nonplanar borane clusters that exhibit higher coordination numbers at boron (e.g., through 3-center 2-electron bonds to bridging hydrides or cations), triboracyclopropenyl-type structures are rings of three boron atoms where substituents at each boron are also coplanar to the ring. Triboracyclopropenyl-containing compounds are extreme cases of inorganic aromaticity. They are the lightest and smallest cyclic structures known to display the bonding and magnetic properties that originate from fully delocalized electrons in orbitals of σ and π symmetry. Although three-membered rings of boron are frequently so highly strained as to be experimentally inaccessible, academic interest in their distinctive aromaticity and possible role as intermediates of borane pyrolysis motivated extensive computational studies by theoretical chemists. Beginning in the late 1980s with mass spectrometry work by Anderson et al. on all-boron clusters, experimental studies of triboracyclopropenyls were for decades exclusively limited to gas-phase investigations of the simplest rings (ions of B3). However, more recent work has stabilized the triboracyclopropenyl moiety via coordination to donor ligands or transition metals, dramatically expanding the scope of its chemistry.
 W
WTrinitrotriazine, or 2,4,6-trinitro-1,3,5-triazine, is a theoretical explosive compound. Synthesis of this compound has been elusive despite its simple structure, as conventional nitration of triazine becomes increasingly more difficult as more nitro groups are added. A successful route would more likely proceed by trimerisation of nitryl cyanide. The precursor, nitryl cyanide, was first synthesized by Rahm et al. in 2014.
 W
W1,2,4-Trioxane is one of the isomers of trioxane. It has the molecular formula C3H6O3 and consists of a six membered ring with three carbon atoms and three oxygen atoms. The two adjacent oxygen atoms form a peroxide functional group and the other forms an ether functional group. It is like a cyclic acetal but with one of the oxygen atoms in the acetal group being replaced by a peroxide group.
 W
WUranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weakly radioactive because all isotopes of uranium are unstable; the half-lives of its naturally occurring isotopes range between 159,200 years and 4.5 billion years. The most common isotopes in natural uranium are uranium-238 and uranium-235. Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70% higher than that of lead, and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite.
 W
WZirconocene is a hypothetical compound with 14 valence electrons, which has not been observed or isolated. It is an organometallic compound consisting of two cyclopentadienyl rings bound on a central zirconium atom. A crucial question in research is what kind of ligands can be used to stabilize the Cp2ZrII metallocene fragment to make it available for further reactions in organic synthesis.