 W
WIn chemistry, a radical is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes.
 W
WIn chemistry, a radical is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes.
 W
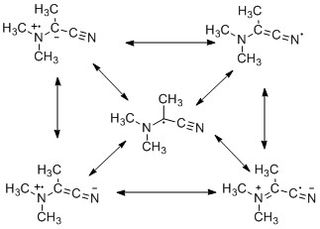
WThe captodative effect is the stabilization of radicals by a synergistic effect of an electron-withdrawing substituent and an electron-donating substituent. The name originates as the electron-withdrawing group (EWG) is sometimes called the "captor" group, whilst the electron-donating group (EDG) is the "dative" substituent. Olefins with this substituent pattern are sometime described as captodative. Radical reactions play an integral role in several chemical reactions and are also important to the field of polymer science.
 W
WChlorine dioxide is a chemical compound with the formula ClO2 that exists as yellowish-green gas above 11 °C, a reddish-brown liquid between 11 °C and −59 °C, and as bright orange crystals below −59 °C. It is an oxidizing agent, able to transfer oxygen to a variety of substrates, while gaining one or more electrons via oxidation-reduction (redox). It does not hydrolyze when it enters water, and is usually handled as a dissolved gas in solution in water. Potential hazards with chlorine dioxide include health concerns, explosiveness and fire ignition. It is commonly used as a bleach.
 W
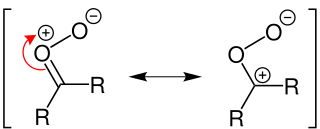
WA Criegee intermediate is a carbonyl oxide with two charge centres. These chemicals may react with sulfur dioxide and nitrogen oxides in the earth's atmosphere, and are implicated in the formation of aerosols, which are an important factor in controlling global climate. Criegee intermediates are also an important source of OH. OH radicals are the most important oxidant in the troposphere, and are important in controlling air quality and pollution.
 W
WThe cyano radical (or cyanido radical) is a radical with molecular formula CN, sometimes written ·CN. The cyano radical was one of the first detected molecules in the interstellar medium, in 1938. Its detection and analysis was influential in astrochemistry. The discovery was confirmed with a coudé spectrograph, which was made famous and credible due to this detection. ·CN has been observed in both diffuse clouds and dense clouds. Usually, CN is detected in regions with hydrogen cyanide, hydrogen isocyanide, and HCNH+, since it is involved in the creation and destruction of these species.
 W
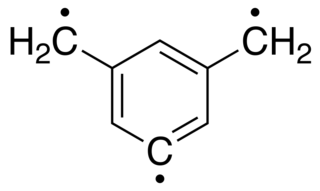
W5-Dehydro-m-xylylene (DMX) is an aromatic organic triradical and the first known organic molecule to violate Hund's Rule.
 W
WDeoxyadenosyl radical is a free radical that is structurally related to adenosine by removal of a 5'-hydroxy group from adenosine. This radical occurs in nature as a reactive intermediate. It is generated by radical SAM enzymes and by some varieties of vitamin B12. The deoxyadenosyl radical abstracts hydrogen atoms from substrates, causing rearrangements and other post transcriptional modifications required for biosynthesis.
 W
WDihydroxymethylidene is a chemical compound with formula C(OH)2. It is an unstable tautomer of formic acid. There is no evidence that this compound exists in solution, but the molecule has been detected in the gas phase. Many related carbenes are known, although they are often transient.
 W
WDistonic ions are chemical species that contain two ionic charges on the same molecule separated by two or more carbon or heteroatoms. Distonic radical ions are unique due to the fact that its charges and radical sites are in different locations, unlike regular radicals where the formal charge and unpaired electron are in the same location. These molecular species are created when ionization takes place with either zwitterions or diradicals; ultimately, a neutral molecule loses an electron. Through experimental research distonic radicals have been found to be extremely stable gas phase ions and can be separated into different classes depending on the inherent features of the charged portion of the ion.
 W
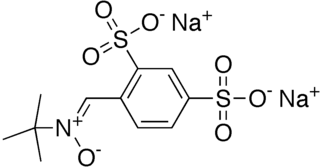
WDisufenton sodium is the disulfonyl derivative of the neuroprotective spin trap phenylbutylnitrone or "PBN". It was under development at the drug company AstraZeneca. A 2005 phase-3 clinical trial called "SAINT-1" reported some efficacy in the acute treatment of ischemia injury due to stroke. However, a 2006 attempt to repeat this trial indicated no significant activity. After ruling out other causes, the authors tentatively attributed the positive results in the first trial to "chance". AstraZeneca then terminated the development programme. PBN and its derivatives hydrolyze and oxidize in vitro to form respectively MNP-OH and its parent spin-trap MNP.
 W
WDPPH is a common abbreviation for the organic chemical compound 2,2-diphenyl-1-picrylhydrazyl. It is a dark-colored crystalline powder composed of stable free-radical molecules. DPPH has two major applications, both in laboratory research: one is a monitor of chemical reactions involving radicals, most notably it is a common antioxidant assay, and another is a standard of the position and intensity of electron paramagnetic resonance signals.
 W
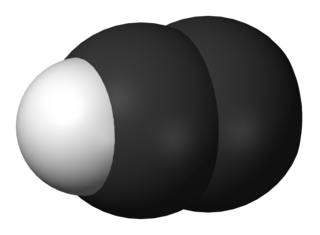
WThe ethynyl radical is an organic compound with the chemical formula C≡CH. It is a simple molecule that does not occur naturally on Earth but is abundant in the interstellar medium. It was first observed by electron spin resonance isolated in a solid argon matrix at liquid helium temperatures in 1963 by Cochran and coworkers at the Johns Hopkins Applied Physics Laboratory. It was first observed in the gas phase by Tucker and coworkers in November 1973 toward the Orion Nebula, using the NRAO 11-meter radio telescope. It has since been detected in a large variety of interstellar environments, including dense molecular clouds, bok globules, star forming regions, the shells around carbon-rich evolved stars, and even in other galaxies.
 W
WFrémy's salt is a chemical compound with the formula (K4[ON(SO3)2]2), sometimes written as (K2[NO(SO3)2]). It a bright yellowish-brown solid, but its aqueous solutions are bright violet. The related sodium salt, i.e. disodium nitrosodisulfonate (NDS, Na2ON(SO3)2, CAS RN 29554-37-8) is also referred to as Frémy's salt.
 W
WGalvinoxyl is a commercially available radical scavenger. It finds use both as a probe for studying radical reactions and as an inhibitor of radical polymerization. It may be synthesized by oxidation of the parent phenol with lead dioxide or potassium hexacyanoferrate(III). Its radical structure is confirmed by the loss of the O-H stretch in the IR spectrum and by electron spin resonance; it is stable even in the presence of oxygen.
 W
WThe hydrocarboxyl radical, HOCO, is an unstable molecular radical important in combustion. It is formed by the reaction of the hydroxyl radical with carbon monoxide. Hydrocarboxyl then breaks up to form carbon dioxide and atomic hydrogen. Much of the carbon dioxide on Earth and Mars has been produced via the hydrocarboxyl radical. HOCO formed from OH and CO initially is in an excited state. It can transfer energy to other molecules such as N2 or other carbon monoxide molecules.
 W
WThe hydroperoxyl radical, also known as the hydrogen superoxide, is the protonated form of superoxide with the chemical formula HO2. This species plays an important role in the atmosphere and as a reactive oxygen species in cell biology.
 W
W4-Hydroxy-TEMPO or TEMPOL, formally 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl, is a heterocyclic compound. Like the related TEMPO, it is used as a catalyst and chemical oxidant by virtue of being a stable aminoxyl radical. Its major appeal over TEMPO is that is less expensive, being produced from triacetone amine, which is itself made via the condensation of acetone and ammonia. This makes it economically viable on an industrial scale.
 W
WThe hydroxyl radical is the diatomic molecule ·OH. The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals is produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also important radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and SCC in coolant systems subjected to radioactive environments.
 W
WImidogen is an inorganic compound with the chemical formula NH. Like other simple radicals, it is highly reactive and consequently short-lived except as a dilute gas. Its behavior depends on its spin multiplicity, i.e. the triplet versus singlet ground state.
 W
WA ketyl group in organic chemistry is an anion radical that contains a group R2C−O•. It is the product of the 1-electron reduction of a ketone.
 W
WThe Koelsch radical is a chemical compound that is an unusually stable carbon-centered radical, due to its resonance structures.
 W
WLithium naphthalene is an organic salt with the chemical formula Li+C10H8−. In the research laboratory, it is used as a reductant in the synthesis of organic, organometallic, and inorganic chemistry. It is usually generated in situ. Lithium naphthalene crystallizes with ligands bound to Li+.
 W
WLutetium phthalocyanine is a coordination compound derived from lutetium and two phthalocyanines. It was the first known example of a molecule that is an intrinsic semiconductor. It exhibits electrochromism, changing color when subject to a voltage.
 W
WMethyl is an organic compound with the chemical formula CH•3. It is a metastable colourless gas, which is mainly produced in situ as a precursor to other hydrocarbons in the petroleum cracking industry. It can act as either a strong oxidant or a strong reductant, and is quite corrosive to metals.
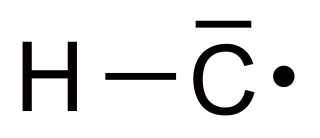 W
WMethylidyne, or (unsubstituted) carbyne, is an organic compound whose molecule consists of a single hydrogen atom bonded to a carbon atom. It is the parent compound of the carbynes, which can be seen as obtained from it by substitution of other functional groups for the hydrogen.
 W
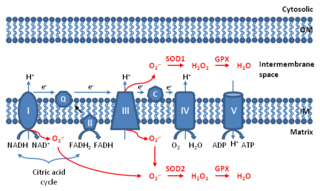
WMitochondrial ROS are reactive oxygen species (ROS) that are produced by mitochondria. Generation of mitochondrial ROS mainly takes place at the electron transport chain located on the inner mitochondrial membrane during the process of oxidative phosphorylation. Leakage of electrons at complex I and complex III from electron transport chains leads to partial reduction of oxygen to form superoxide. Subsequently, superoxide is quickly dismutated to hydrogen peroxide by two dismutases including superoxide dismutase 2 (SOD2) in mitochondrial matrix and superoxide dismutase 1 (SOD1) in mitochondrial intermembrane space. Collectively, both superoxide and hydrogen peroxide generated in this process are considered as mitochondrial ROS.
 W
WTrioxidonitrogen(•) or nitrate radical is an oxide of nitrogen with formula NO3, consisting of three oxygen atoms covalently bound to a nitrogen atom. This highly unstable blue compound has not been isolated in pure form, but can be generated and observed as a short-lived component of gas, liquid, or solid systems.
 W
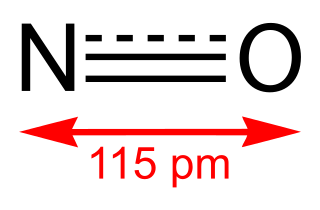
WNitric oxide is a colorless gas with the formula NO. It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula. Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding.
 W
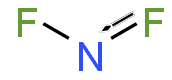
WNitrogen difluoride, also known as difluoroamino is a reactive radical molecule with formula NF2. This small molecule is in equilibrium with its dimer dinitrogen tetrafluoride.N2F4 ⇌ 2 NF2•
 W
WNitrogen dioxide is a chemical compound with the formula NO2. It is one of several nitrogen oxides. NO2 is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the production of fertilizers. At higher temperatures it is a reddish-brown gas. It can be fatal if inhaled in large quantity. Nitrogen dioxide is a paramagnetic, bent molecule with C2v point group symmetry.
 W
WNitrogen dioxide poisoning is the illness resulting from the toxic effect of nitrogen dioxide. It usually occurs after the inhalation of the gas beyond the threshold limit value. Nitrogen dioxide is reddish-brown with a very harsh smell at high concentrations, at lower concentrations it is colorless but may still have a harsh odour. Nitrogen dioxide poisoning depends on the duration, frequency, and intensity of exposure.
 W
WNitryl is the nitrogen dioxide (NO2) moiety when it occurs in a larger compound as a univalent fragment. Examples include nitryl fluoride (NO2F) and nitryl chloride (NO2Cl).
 W
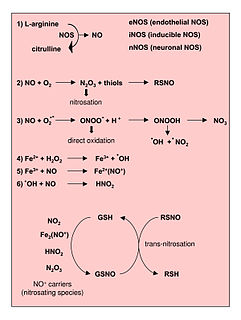
WReactive nitrogen species (RNS) are a family of antimicrobial molecules derived from nitric oxide (•NO) and superoxide (O2•−) produced via the enzymatic activity of inducible nitric oxide synthase 2 (NOS2) and NADPH oxidase respectively. NOS2 is expressed primarily in macrophages after induction by cytokines and microbial products, notably interferon-gamma (IFN-γ) and lipopolysaccharide (LPS).
 W
WReactive oxygen species (ROS) are highly reactive chemicals formed from O2. Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
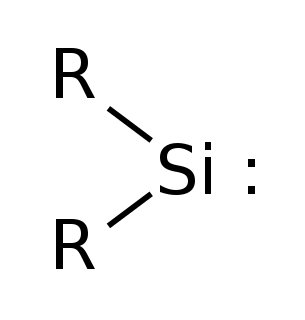 W
WSilylene is a chemical compound with the formula SiH2. It is the silicon analog of methylene, the simplest carbene. Silylene is a stable molecule as a gas but rapidly reacts in a bimolecular manner when condensed. Unlike carbenes, which can exist in the singlet or triplet state, silylene (and all of its derivatives) are singlets.
 W
WSodium naphthalene is an organic salt with the chemical formula Na+C10H8−. In the research laboratory, it is used as a reductant in the synthesis of organic, organometallic, and inorganic chemistry. It is usually generated in situ. When isolated, it invariably crystallizes as a solvate with ligands bound to Na+.
 W
WA spin label (SL) is an organic molecule which possesses an unpaired electron, usually on a nitrogen atom, and the ability to bind to another molecule. Spin labels are normally used as tools for probing proteins or biological membrane-local dynamics using electron paramagnetic resonance spectroscopy. The site-directed spin labeling (SDSL) technique allows one to monitor a specific region within a protein. In protein structure examinations, amino acid-specific SLs can be used.
 W
WSulfur trifluoride is the inorganic chemical compound with the formula SF3. It is a radical.
 W
WA superoxide is a compound that contains the superoxide ion, which has the chemical formula O−2. The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature. Molecular oxygen (dioxygen) is a diradical containing two unpaired electrons, and superoxide results from the addition of an electron which fills one of the two degenerate molecular orbitals, leaving a charged ionic species with a single unpaired electron and a net negative charge of −1. Both dioxygen and the superoxide anion are free radicals that exhibit paramagnetism.
 W
W(2,2,6,6-Tetramethylpiperidin-1-yl)oxyl or (2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl, commonly known as TEMPO, is a chemical compound with the formula (CH2)3(CMe2)2NO. This heterocyclic compound is a red-orange, sublimable solid. As a stable aminoxyl radical, it has applications in chemistry and biochemistry. TEMPO is used as a radical marker, as a structural probe for biological systems in conjunction with electron spin resonance spectroscopy, as a reagent in organic synthesis, and as a mediator in controlled radical polymerization.
 W
WTriangulene is the smallest triplet-ground-state polybenzenoid. It exists as a biradical with the chemical formula C22H12. It was first hypothesized by Czech chemist Erich Clar in 1953. Its first confirmed synthesis was published in a February 2017 issue of Nature Nanotechnology, in a project led by researchers David Fox and Anish Mistry at the University of Warwick in collaboration with IBM. Other attempts by Japanese researchers have been successful only in making substituted triangulene derivatives.
 W
WTrimethylenemethane is a chemical compound with formula C4H6. It is a neutral free molecule with two unsatisfied valence bonds, and is therefore a highly reactive free radical. Formally, it can be viewed as an isobutylene molecule C4H8 with two hydrogen atoms removed from the terminal methyl groups.
 W
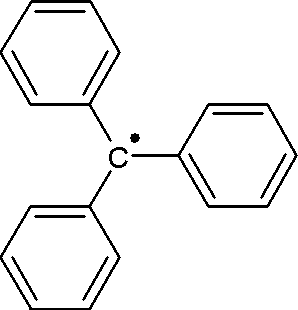
WThe triphenylmethyl radical is a persistent radical and the first radical ever described in organic chemistry.
 W
WTris(4-bromophenyl)ammoniumyl hexachloroantimonate is the organic compound with the formula [(4-BrC6H4)3N]SbCl6. Commonly known as magic blue, it is the hexachloroantimonate salt of an amine radical cation. It is a blue solid that reacts with many solvents but is soluble in acetonitrile. The compound is a popular oxidizing agent in organic and organometallic chemistry, with a reduction potential of 0.67 V vs. ferrocene/ferrocenium (MeCN solution) or 0.70 V vs. ferrocene/ferrocenium (dichloromethane solution).