 W
WA refrigerant is a working fluid used in the refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are heavily regulated due to their toxicity, flammability and the contribution of CFC and HCFC refrigerants to ozone depletion and that of HFC refrigerants to climate change.
 W
WAmmonia is a compound of nitrogen and hydrogen with the formula NH3. A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. It is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45 percent of the world's food and fertilizers. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water.
 W
WBrine is a high-concentration solution of salt (NaCl) in water (H2O). In diverse contexts, brine may refer to the salt solutions ranging from about 3.5% (a typical concentration of seawater, on the lower end of that of solutions used for brining foods) up to about 26% (a typical saturated solution, depending on temperature). Brine forms naturally due to evaporation of ground saline water but it is also generated in the mining of sodium chloride. Brine is used for food processing and cooking (pickling and brining), for de-icing of roads and other structures, and in a number of technological processes. It is also a by-product of many industrial processes, such as desalination, so it requires wastewater treatment for proper disposal or further utilization (fresh water recovery).
 W
WBromodifluoromethane or Halon 1201 or FC-22B1 is a gaseous trihalomethane or a hydrobromofluorocarbon.
 W
WButane or n-butane is an alkane with the formula C4H10. Butane is a gas at room temperature and atmospheric pressure. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature. The name butane comes from the roots but- (from butyric acid, named after the Greek word for butter) and -ane. It was discovered by the chemist Edward Frankland in 1849. It was found dissolved in crude petroleum in 1864 by Edmund Ronalds, who was the first to describe its properties.
 W
WCarbon dioxide is an acidic colorless gas with a density about 53% higher than that of dry air. Carbon dioxide molecules consist of a carbon atom covalently double bonded to two oxygen atoms. It occurs naturally in Earth's atmosphere as a trace gas. The current concentration is about 0.04% (412 ppm) by volume, having risen from pre-industrial levels of 280 ppm. Natural sources include volcanoes, hot springs and geysers, and it is freed from carbonate rocks by dissolution in water and acids. Because carbon dioxide is soluble in water, it occurs naturally in groundwater, rivers and lakes, ice caps, glaciers and seawater. It is present in deposits of petroleum and natural gas. Carbon dioxide has a sharp and acidic odor and generates the taste of soda water in the mouth. However, at normally encountered concentrations it is odorless.
 W
WCarbon tetrachloride, also known by many other names (such as tetrachloromethane, also recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVACR) is an organic compound with the chemical formula CCl4. It is a colourless liquid with a "sweet" smell that can be detected at low levels. It is practically not flammable at lower temperatures. It was formerly widely used in fire extinguishers, as a precursor to refrigerants and as a cleaning agent, but has since been phased out because of environmental and safety concerns. Exposure to high concentrations of carbon tetrachloride (including vapor) can affect the central nervous system and degenerate the liver and kidneys. Prolonged exposure can be fatal.
 W
WTetrafluoromethane, also known as carbon tetrafluoride or R-14, is the simplest perfluorocarbon (CF4). As its IUPAC name indicates, tetrafluoromethane is the perfluorinated counterpart to the hydrocarbon methane. It can also be classified as a haloalkane or halomethane. Tetrafluoromethane is a useful refrigerant but also a potent greenhouse gas. It has a very high bond strength due to the nature of the carbon–fluorine bond.
 W
W1-Chloro-1,1-difluoroethane (HCFC-142b) is a haloalkane with the chemical formula CH3CClF2. It belongs to the hydrochlorofluorocarbon (HCFC) family of man-made compounds that contribute significantly to both ozone depletion and global warming when released into the environment. It is primarily used as a refrigerant where it is also known as R-142b and by trade names including Freon-142b.
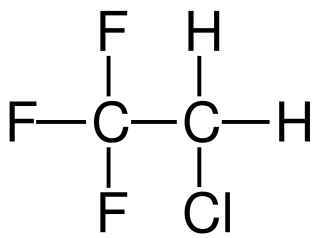 W
W2-Chloro-1,1,1-trifluoroethane, also known as 1,1,1-trifluoro-2-chloroethane or Freon 133a, is an alkyl halide belonging to the category of chlorofluorocarbons, having chemical formula F3C-CH2-Cl. Under standard conditions it appears as a colorless gas, partially soluble in water. It is used as a refrigerant, as a solvent and as a reagent in organic synthesis.
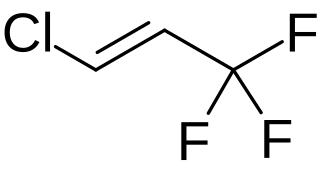 W
W1-Chloro-3,3,3-trifluoropropene (HFO-1233zd) is the unsaturated chlorofluorocarbon with the formula HClC=C(H)CF3. This colorless gas is of interest as a more environmentally friendly (lower GWP; global warming potential) refrigerant in air conditioners. The compound exists as E- and Z-isomers. It is prepared by fluorination and dehydrohalogenation reactions starting with 1,1,1,3,3-pentachloropropane.
 W
WChlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated paraffin hydrocarbons that contain only carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and propane. They are also commonly known by the DuPont brand name Freon.
 W
WChlorofluoromethane or Freon 31 is the hydrochlorofluorocarbon (HCFC) with the formula CH2ClF. It is a colorless, odorless, flammable gas.
 W
WChloropentafluoroethane is a chlorofluorocarbon (CFC) once used as a refrigerant and also known as R-115 and CFC-115. Its production and consumption has been banned since 1 January 1996 under the Montreal Protocol because of its high ozone depletion potential and very long lifetime when released into the environment. CFC-115 is also a potent greenhouse gas.
 W
W W
W1,1-Dichloro-1-fluoroethane is a haloalkane with the formula C2H3Cl2F. It is one of the three isomers of dichlorofluoroethane. It belongs to the hydrochlorofluorocarbon (HCFC) family of man-made compounds that contribute significantly to both ozone depletion and global warming when released into the environment.
 W
WDichlorofluoromethane or Freon 21 or R 21 is a halomethane or hydrochlorofluorocarbon with the formula CHCl2F. It is a colorless and odorless gas. It is produced by fluorination of chloroform using a catalyst such as antimony trifluoride:CHCl3 + HF → CHCl2F + HCl
 W
WDichloromethane (DCM or methylene chloride) is an organochloride compound with the formula CH2Cl2. This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with water, it is polar, and miscible with many organic solvents.
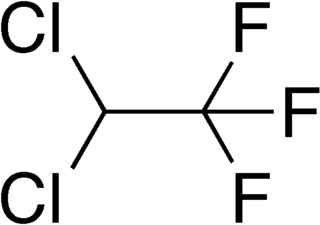 W
W2,2-Dichloro-1,1,1-trifluoroethane or HCFC-123 is considered as an alternative to CFC-11 in low pressure refrigeration and HVAC systems, and should not be used in foam blowing processes or solvent applications.
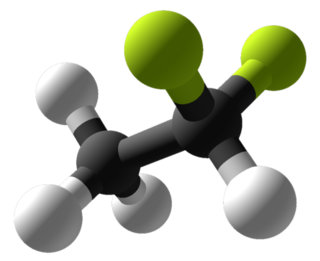 W
W1,1-Difluoroethane, or DFE, is an organofluorine compound with the chemical formula C2H4F2. This colorless gas is used as a refrigerant, where it is often listed as R-152a (refrigerant-152a) or HFC-152a (hydrofluorocarbon-152a). It is also used as a propellant for aerosol sprays and in gas duster products. As an alternative to chlorofluorocarbons, it has an ozone depletion potential of zero, a lower global warming potential (124) and a shorter atmospheric lifetime (1.4 years).
 W
WDry ice is the solid form of carbon dioxide. It is used primarily as a cooling agent, but is also used in fog machines at theatres for dramatic effects. Its advantages include lower temperature than that of water ice and not leaving any residue. It is useful for preserving frozen foods where mechanical cooling is unavailable.
 W
WFluoromethane, also known as methyl fluoride, Freon 41, Halocarbon-41 and HFC-41, is a non-toxic, liquefiable, and flammable gas at standard temperature and pressure. It is made of carbon, hydrogen, and fluorine. The name stems from the fact that it is methane (CH4) with a fluorine atom substituted for one of the hydrogen atoms. It is used in semiconductor manufacturing processes as an etching gas in plasma etch reactors.
 W
WThe haloalkanes are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.
 W
W1,1,1,2,3,3,3-Heptafluoropropane, also called heptafluoropropane, HFC-227ea, HFC-227 or FM200, as well as apaflurane (INN), is a colourless, odourless gaseous halocarbon commonly used as a gaseous fire suppression agent.
 W
WHexafluoroethane is the perfluorocarbon counterpart to the hydrocarbon ethane. It is a non-flammable gas negligibly soluble in water and slightly soluble in alcohol. It is an extremely potent and long-lived greenhouse gas.
 W
W1,1,1,3,3,3-Hexafluoropropane is an organic chemical, an organofluoride. It is a colorless gas, usually available in the form of a liquid gas. It is used as a fire suppression agent, a foaming agent, a highly effective refrigerant, a heat transfer medium, a dielectric gas, a sterilant carrier, a polymerization medium, a carrier fluid, a displacement drying agent, a thermodynamic power cycle working fluid, etc. It is used as a cold gas rocket propellant by the Mars Cube One spacecraft.
 W
WHydrofluoroolefins (HFOs) are unsaturated organic compounds composed of hydrogen, fluorine and carbon. These organofluorine compound are of interest as refrigerants. Unlike traditional hydrofluorocarbons (HFCs) and chlorofluorocarbons (CFCs), which are saturated, HFOs are olefins, otherwise known as alkenes.
 W
WHydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula H2. It is colorless, odorless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons.
 W
WPropane is a three-carbon alkane with the molecular formula C3H8. It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel in domestic and industrial applications and in low-emissions public transportation. Discovered in 1857 by the French chemist Marcellin Berthelot, it became commercially available in the US by 1911. Propane is one of a group of liquefied petroleum gases (LP gases). The others include butane, propylene, butadiene, butylene, isobutylene, and mixtures thereof. Propane has a lower energy density but burns more cleanly than gasoline and coal.
 W
WNeon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered in 1898 as one of the three residual rare inert elements remaining in dry air, after nitrogen, oxygen, argon and carbon dioxide were removed. Neon was the second of these three rare gases to be discovered and was immediately recognized as a new element from its bright red emission spectrum. The name neon is derived from the Greek word, νέον, neuter singular form of νέος (neos), meaning new. Neon is chemically inert, and no uncharged neon compounds are known. The compounds of neon currently known include ionic molecules, molecules held together by van der Waals forces and clathrates.
 W
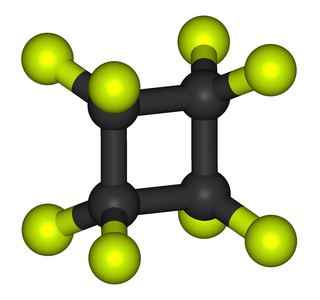
WOctafluorocyclobutane, or perfluorocyclobutane, C4F8, is an organofluorine compound which enjoys several niche applications. Octafluorocyclobutane is a colourless gas and shipped as a liquefied gas. It is the perfluorinated analogue of cyclobutane whereby all C–H bonds are replaced with C–F bonds.
 W
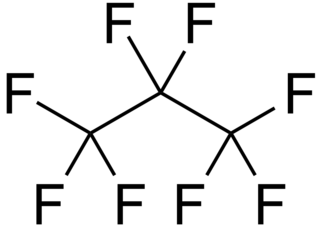
WOctafluoropropane (C3F8) is the perfluorocarbon counterpart to the hydrocarbon propane. This non-flammable synthetic material has applications in semiconductor production and medicine. It is also an extremely potent greenhouse gas.
 W
W1,1,1,3,3-Pentafluoropropane (HFC-245fa) is a hydrofluorocarbon is a colorless gas used primarily for closed-cell spray foam insulation. HFC-245fa is also known as pentafluoropropane and by its chemical name 1,1,1,3,3-pentafluoropropane.
 W
W1,2,3,3,3-Pentafluoropropene is the unsaturated fluorocarbon with the formula HFC=C(F)CF3. This colorless gas is of interest as a precursor to hydrofluoroolefins (HFOs), which are used as refrigerants in air conditioners. Of the methods reported for its synthesis, one route involves dehydrofluorination of 1,1,2,3,3,3-hexafluoropropane. The compound exists as a mixture of E- and Z-isomers.
 W
WPropane is a three-carbon alkane with the molecular formula C3H8. It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel in domestic and industrial applications and in low-emissions public transportation. Discovered in 1857 by the French chemist Marcellin Berthelot, it became commercially available in the US by 1911. Propane is one of a group of liquefied petroleum gases (LP gases). The others include butane, propylene, butadiene, butylene, isobutylene, and mixtures thereof. Propane has a lower energy density but burns more cleanly than gasoline and coal.
 W
WSlurry ice is a phase changing refrigerant made up of millions of ice "micro-crystals" formed and suspended within a solution of water and a freezing point depressant. Some compounds used in the field are salt, ethylene glycol, propylene glycol, alcohols like isobutyl and ethanol, and sugars like sucrose and glucose. Slurry ice has greater heat absorption compared to single phase refrigerants like brine, because the melting enthalpy of the ice is also used.
 W
WSulfur dioxide or sulphur dioxide is the chemical compound with the formula SO2. It is a toxic gas responsible for the smell of burnt matches. It is released naturally by volcanic activity and is produced as a by-product of copper extraction and the burning of sulfur-bearing fossil fuels. Sulfur dioxide has a pungent smell like nitric acid.
 W
WSulfur hexafluoride (SF6) or sulphur hexafluoride (British spelling) is an extremely potent and persistent greenhouse gas that is primarily utilized as an electrical insulator and arc suppressant. It is inorganic, colorless, odorless, non-flammable, and non-toxic. SF6 has an octahedral geometry, consisting of six fluorine atoms attached to a central sulfur atom. It is a hypervalent molecule.
 W
W1,1,2,2-Tetrachloroethane is a chlorinated derivative of ethane. It has the highest solvent power of any chlorinated hydrocarbon. As a refrigerant, it is used under the name R-130.
 W
W1,3,3,3-Tetrafluoropropene (HFO-1234ze(E), R-1234ze) is a hydrofluoroolefin. It was developed as a "fourth generation" refrigerant to replace fluids such as R-134a, as a blowing agent for foam and aerosol applications, and in air horns and gas dusters. The use of R-134a is being phased out because of its high global-warming potential. HFO-1234ze(E) itself has zero ozone-depletion potential (ODP=0), a very low global-warming potential (GWP < 1 ), even lower than CO2, and it is classified by ANSI/ASHRAE as class A2L refrigerant (lower flammability and lower toxicity). In open atmosphere however, HFO-1234ze actually might form HFC-23 as one of its secondary atmospheric breakdown products. HFC-23 is a very potent greenhouse gas with a GWP100 of 14,800. The secondary GWP of R-1234ze would then be in the range of 1,400±700 considering the amount of HFC-23 which may form from HFO-1234ze in the atmosphere. Besides the global warming potential, when HFOs decompose in the atmosphere, trifluoroacetic acid (TFA(A)) is formed, which also remains in the atmosphere for several days. The trifluoroacetic acid then forms trifluoroacetate (TFA), a salt of trifluoroacetic acid, in water and on the ground. Due to its high polarity and low degradability, it is difficult to remove TFA from drinking water (ICPR 2019).
 W
W2,3,3,3-Tetrafluoropropene, HFO-1234yf, is a hydrofluoroolefin (HFO) with the formula CH2=CFCF3. It is also designated R-1234yf as the first of a new class of refrigerants: it is marketed under the name Opteon YF by Chemours and as Solstice YF by Honeywell.
 W
W1,1,1-Trifluoroethane, or R-143a or simply trifluoroethane, is a hydrofluorocarbon (HFC) compound that is a colorless gas. It should not be confused with the much more commonly used HFC gas R-134a, nor confused with the isomeric compound 1,1,2-trifluoroethane. 1,1,1-Trifluoroethane has a critical temperature of 73 °C.
 W
WVinyl fluoride is an organic halide with the chemical formula C2H3F. It is a colorless gas with a faint etherlike odor. It is used as the monomeric precursor to the fluoropolymer polyvinylfluoride.