 W
WA hinged expansion joint is a metallic assembly, that can rotate in a single plane, used to absorb changes resulting from piping thermal expansion or contraction.
 W
WA refractory material or refractory is a material that is resistant to decomposition by heat, pressure, or chemical attack, and retains strength and form at high temperatures. Refractories are polycrystalline, polyphase, inorganic, non-metallic, porous, and heterogeneous. They are typically composed of oxides or carbides, nitrides etc. of the following materials: silicon, aluminium, magnesium, calcium, and zirconium. Some metals with melting points >1850 °C like niobium, chromium, zirconium, tungsten, rhenium, tantalum, molybdenum etc. are also considered refractories.
 W
WA Refractory lined expansion joint is an assembly used in a pipe line to allow it to expand and contract as climate conditions move from hot to cold and helps to ensure that the system remains functional. The refractory-lining can be vibra cast insulation with anchors, abrasion resistant refractory in hex mesh, gunned insulating refractory, or poured insulating refractory. Refractory lined expansion joints can be hinged, in-line pressure balanced, gimbal, tied-universal depending on the temperature, pressure, movement and flow media conditions.
 W
WA Toroidal expansion joint is a metallic assembly that consists of a series of toroidal convolutions which are circular tubes wrapped around pipe ends or weld ends and have a gap at the inside diameter to allow for axial stroke while absorbing changes in expansion or contraction of the pipe line. Convolutions are the portion of the bellows that allow it to be flexible. The convolutions are formed around reinforcing bands so that only the concave portion of the torus allows for flexibility. Toroidal expansion joints are typically used in high pressure applications, where little movement is required, and generally used for heat exchangers. Usually, they are hydraulically formed, but others are free formed. These expansion joints are also referred to as "Omega" bellows due to their shape resembling the Greek letter, Omega.
 W
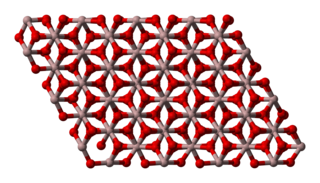
WAluminium oxide is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium(III) oxide. It is commonly called alumina and may also be called aloxide, aloxite, or alundum depending on particular forms or applications. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire. Al2O3 is significant in its use to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point.
 W
WBeryllium nitride, Be3N2, is a nitride of beryllium. It can be prepared from the elements at high temperature (1100–1500 °C), unlike Beryllium azide or BeN6, it decomposes in vacuum into beryllium and nitrogen. It is readily hydrolysed forming beryllium hydroxide and ammonia. It has two polymorphic forms cubic α-Be3N2 with a defect anti-fluorite structure, and hexagonal β-Be3N2. It reacts with silicon nitride, Si3N4 in a stream of ammonia at 1800–1900 °C to form BeSiN2.
 W
WCalcium aluminates are a range of materials obtained by heating calcium oxide and aluminium oxide together at high temperatures. They are encountered in the manufacture of refractories and cements.
 W
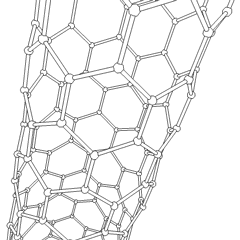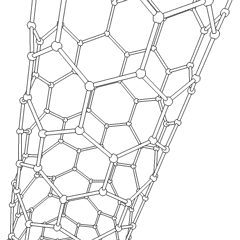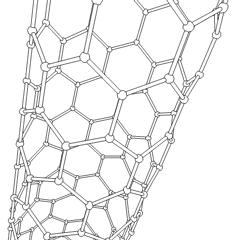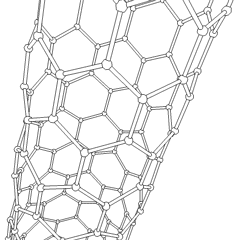
WA carbon nanothread is a sp3-bonded, one-dimensional carbon crystalline nanomaterial. The tetrahedral sp3-bonding of its carbon is similar to that of diamond. Nanothreads are only a few atoms across, more than 20,000 times thinner than a human hair. They consist of a stiff, strong carbon core surrounded by hydrogen atoms. Carbon nanotubes, although also one-dimensional nanomaterials, in contrast have sp2-carbon bonding as is found in graphite. The smallest carbon nanothread has a diameter of only 0.2 nanometer, much smaller than the diameter of a single-wall carbon nanotube.
 W
WCarbon nanotubes (CNTs) are tubes made of carbon with diameters typically measured in nanometers.
 W
WCerium hexaboride (CeB6, also called cerium boride, CeBix, CEBIX, and (incorrectly) CeB) is an inorganic chemical, a boride of cerium. It is a refractory ceramic material. It has a low work function, one of the highest electron emissivities known, and is stable in vacuum. The principal use of cerium hexaboride is a coating of hot cathodes. It usually operates at temperature of 1450 °C.
 W
WChromite is a crystalline mineral composed primarily of iron(II) oxide and chromium(III) oxide compounds. It can be represented by the chemical formula of FeCr2O4. It is an oxide mineral belonging to the spinel group. The element magnesium can substitute for iron in variable amounts as it forms a solid solution with magnesiochromite (MgCr2O4). A substitution of the element aluminium can also occur, leading to hercynite (FeAl2O4). Chromite today is mined particularly to make stainless steel through the production of ferrochrome (FeCr), which is an iron-chromium alloy.
 W
WChromium(II) carbide is a ceramic compound that exists in several chemical compositions: Cr3C2, Cr7C3, and Cr23C6. At standard conditions it exists as a gray solid. It is extremely hard and corrosion resistant. It is also a refractory compound, which means that it retains its strength at high temperatures as well. These properties make it useful as an additive to metal alloys. When chromium carbide crystals are integrated into the surface of a metal it improves the wear resistance and corrosion resistance of the metal, and maintains these properties at elevated temperatures. The hardest and most commonly used composition for this purpose is Cr3C2.
 W
WCubic zirconia (CZ) is the cubic crystalline form of zirconium dioxide (ZrO2). The synthesized material is hard and usually colorless, but may be made in a variety of different colors. It should not be confused with zircon, which is a zirconium silicate (ZrSiO4). It is sometimes erroneously called cubic zirconium.
 W
WDSF Refractories and Minerals Limited is the last major British producer of refractory products, both shaped and unshaped. DSF is located in Friden within the Derbyshire's Peak National Park.
 W
WA fire brick, firebrick, or refractory is a block of ceramic material used in lining furnaces, kilns, fireboxes, and fireplaces. A refractory brick is built primarily to withstand high temperature, but will also usually have a low thermal conductivity for greater energy efficiency. Usually dense firebricks are used in applications with extreme mechanical, chemical, or thermal stresses, such as the inside of a wood-fired kiln or a furnace, which is subject to abrasion from wood, fluxing from ash or slag, and high temperatures. In other, less harsh situations, such as in an electric- or natural gas-fired kiln, more porous bricks, commonly known as "kiln bricks", are a better choice. They are weaker, but they are much lighter and easier to form and insulate far better than dense bricks. In any case, firebricks should not spall, and their strength should hold up well during rapid temperature changes.
 W
WFire clay is a range of refractory clays used in the manufacture of ceramics, especially fire brick. The United States Environmental Protection Agency defines fire clay very generally as a "mineral aggregate composed of hydrous silicates of aluminium (Al2O3·2SiO2·2H2O) with or without free silica."
 W
WGraphite, archaically referred to as plumbago, is a crystalline form of the element carbon with its atoms arranged in a hexagonal structure. It occurs naturally in this form and is the most stable form of carbon under standard conditions. Under high pressures and temperatures it converts to diamond. Graphite is used in pencils and lubricants. It is a good conductor of heat and electricity. Its high conductivity makes it useful in electronic products such as electrodes, batteries, and solar panels.
 W
WHafnium carbide (HfC) is a chemical compound of hafnium and carbon. With a melting point of about 3900 °C, it is one of the most refractory binary compounds known. However, it has a low oxidation resistance, with the oxidation starting at temperatures as low as 430 °C.
 W
WLanthanum hexaboride (LaB6, also called lanthanum boride and LaB) is an inorganic chemical, a boride of lanthanum. It is a refractory ceramic material that has a melting point of 2210 °C, and is insoluble in water and hydrochloric acid. It has a low work function and one of the highest electron emissivities known, and is stable in vacuum. Stoichiometric samples are colored intense purple-violet, while boron-rich ones (above LaB6.07) are blue. Ion bombardment changes its color from purple to emerald green. LaB6 is a superconductor with a relatively low transition temperature of 0.45 K.
 W
WMagnesium oxide (MgO), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture.
 W
WMolybdenum disilicide (MoSi2, or molybdenum silicide), an intermetallic compound, a silicide of molybdenum, is a refractory ceramic with primary use in heating elements. It has moderate density, melting point 2030 °C, and is electrically conductive. At high temperatures it forms a passivation layer of silicon dioxide, protecting it from further oxidation. The thermal stability of MoSi2 alongside its high emissivity make this material, alongside WSi2 attractive for applications as a high emissivity coatings in heat shields for atmospheric entry. MoSi2 is a gray metallic-looking material with tetragonal crystal structure (alpha-modification); its beta-modification is hexagonal and unstable. It is insoluble in most acids but soluble in nitric acid and hydrofluoric acid.
 W
WGraphite, archaically referred to as plumbago, is a crystalline form of the element carbon with its atoms arranged in a hexagonal structure. It occurs naturally in this form and is the most stable form of carbon under standard conditions. Under high pressures and temperatures it converts to diamond. Graphite is used in pencils and lubricants. It is a good conductor of heat and electricity. Its high conductivity makes it useful in electronic products such as electrodes, batteries, and solar panels.
 W
WPyrolytic carbon is a material similar to graphite, but with some covalent bonding between its graphene sheets as a result of imperfections in its production.
 W
WCarbon fibre reinforced carbon (CFRC), carbon–carbon (C/C), or reinforced carbon–carbon (RCC) is a composite material consisting of carbon fiber reinforcement in a matrix of graphite. It was developed for the reentry vehicles of intercontinental ballistic missiles, and is most widely known as the material for the nose cone and wing leading edges of the Space Shuttle orbiter. Carbon-carbon brake discs and brake pads have been the standard component of the brake systems of Formula One racing cars since 1976.
 W
WSaint-Gobain SEFPRO, founded in 1929, produces refractories for the glass industry. The company consists of plants, sales offices and Research and Development Centers employing over 2200 people across four continents, with headquarters in Le Pontet, Vaucluse, France. The group belongs to the ‘Innovative Materials’ division of the Saint-Gobain group.
 W
WSilicon carbide (SiC), also known as carborundum, is a semiconductor containing silicon and carbon. It occurs in nature as the extremely rare mineral moissanite. Synthetic SiC powder has been mass-produced since 1893 for use as an abrasive. Grains of silicon carbide can be bonded together by sintering to form very hard ceramics that are widely used in applications requiring high endurance, such as car brakes, car clutches and ceramic plates in bulletproof vests. Electronic applications of silicon carbide such as light-emitting diodes (LEDs) and detectors in early radios were first demonstrated around 1907. SiC is used in semiconductor electronics devices that operate at high temperatures or high voltages, or both. Large single crystals of silicon carbide can be grown by the Lely method and they can be cut into gems known as synthetic moissanite.
 W
WSilicon dioxide, also known as silica, is an oxide of silicon with the chemical formula SiO2, most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as a synthetic product. Notable examples include fused quartz, fumed silica, silica gel, and aerogels. It is used in structural materials, microelectronics (as an electrical insulator), and as components in the food and pharmaceutical industries.
 W
WTantalcarbide is a rare mineral of tantalum carbide with formula TaC. With a molecular weight of 192.96 g/mol, its primary constituents are tantalum (93.78%) and carbon (6.22%), and has an isometric crystal system. It generally exhibits a bronze or brown to yellow color. On the Mohs hardness scale it registers as a 6-7. Tantalcarbide is generally found in a granular state. It is extremely dense at 14.6 g/. Sub-conchoidal fracturing is exhibited.
 W
WTantalum carbides (TaC) form a family of binary chemical compounds of tantalum and carbon with the empirical formula TaCx, where x usually varies between 0.4 and 1. They are extremely hard, brittle, refractory ceramic materials with metallic electrical conductivity. They appear as brown-gray powders, which are usually processed by sintering.
 W
WThorium dioxide (ThO2), also called thorium(IV) oxide, is a crystalline solid, often white or yellow in color. Also known as thoria, it is produced mainly as a by-product of lanthanide and uranium production. Thorianite is the name of the mineralogical form of thorium dioxide. It is moderately rare and crystallizes in an isometric system. The melting point of thorium oxide is 3300 °C – the highest of all known oxides. Only a few elements (including tungsten and carbon) and a few compounds (including tantalum carbide) have higher melting points. All thorium compounds are radioactive because there are no stable isotopes of thorium.
 W
WTitanium carbide, TiC, is an extremely hard refractory ceramic material, similar to tungsten carbide. It has the appearance of black powder with the sodium chloride crystal structure.
 W
WVanadium carbide is the inorganic compound with the formula VC. It is an extremely hard refractory ceramic material. With a hardness of 9-9.5 Mohs, it is possibly the hardest metal-carbide known. It is of interest because it is prevalent in vanadium metal and alloys.
 W
WYttria-stabilized zirconia (YSZ) is a ceramic in which the cubic crystal structure of zirconium dioxide is made stable at room temperature by an addition of yttrium oxide. These oxides are commonly called "zirconia" (Zr O2) and "yttria" (Y2O3), hence the name.
 W
WZircon ( or ) is a mineral belonging to the group of nesosilicates and is a source of the metal zirconium. Its chemical name is zirconium silicate, and its corresponding chemical formula is ZrSiO4. A common empirical formula showing some of the range of substitution in zircon is (Zr1–y, REEy)(SiO4)1–x(OH)4x–y. Zircon forms in silicate melts with large proportions of high field strength incompatible elements. For example, hafnium is almost always present in quantities ranging from 1 to 4%. The crystal structure of zircon is tetragonal crystal system. The natural color of zircon varies between colorless, yellow-golden, red, brown, blue, and green.
 W
WZirconium carbide (ZrC) is an extremely hard refractory ceramic material, commercially used in tool bits for cutting tools. It is usually processed by sintering.
 W
WZirconium dioxide, sometimes known as zirconia, is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant stabilized cubic structured zirconia, cubic zirconia, is synthesized in various colours for use as a gemstone and a diamond simulant.
 W
WZirconium nitride (ZrN) is an inorganic compound used in a variety of ways due to its properties.
 W
WZirconium silicate, also zirconium orthosilicate, ZrSiO4, is a chemical compound, a silicate of zirconium. It occurs in nature as zircon, a silicate mineral. Powdered zirconium silicate is also known as zircon flour.