 W
WAbsinthin is a naturally produced triterpene lactone from the plant Artemisia absinthium (Wormwood). It constitutes one of the most bitter chemical agents responsible for absinthe's distinct taste. The compound shows biological activity and has shown promise as an anti-inflammatory agent, and should not to be confused with thujone, a neurotoxin also found in Artemisia absinthium.
 W
WAloin, also known as barbaloin, is a bitter, yellow-brown colored compound noted in the exudate of at least 68 Aloe species at levels from 0.1 to 6.6% of leaf dry weight, and in another 17 species at indeterminate levels [Reynolds, 1995b]. It is used as a stimulant-laxative, treating constipation by inducing bowel movements. The compound is present in what is commonly referred to as the aloe latex that exudes from cells adjacent to the vascular bundles, found under the rind of the leaf and in between it and the gel. When dried, it has been used as a bittering agent in commerce [21 CFR 172.510. Scientific names given include Aloe perryi, A. barbadensis, A. ferox, and hybrids of A. ferox with A. africana and A. spicata.]. Aloe is listed in federal regulations as a natural substance that may be "safely used in food" when used "in the minimum quantity required to produce their intended physical or technical effect and in accordance with all the principles of good manufacturing practice." This food application is generally limited to use in quite small quantities as a flavoring in alcoholic beverages and may usually be identified only as a "natural flavor."
 W
WAlpha acids are a class of chemical compounds primarily of importance to the production of beer. They are found in the resin glands of the flowers of the hop plant and are the source of hop bitterness.
 W
WAmarogentin is a chemical compound found in gentian or in Swertia chirata.
 W
WAndrographolide is a labdane diterpenoid that has been isolated from the stem and leaves of Andrographis paniculata. Andrographolide is an extremely bitter substance.
 W
WDenatonium, usually available as denatonium benzoate and as denatonium saccharide (BITTERANT-s), is the most bitter chemical compound known, with bitterness thresholds of 0.05 ppm for the benzoate and 0.01 ppm for the saccharide. It was discovered in 1958 during research on local anesthetics by MacFarlan Smith of Edinburgh, Scotland, and registered under the trademark Bitrex.
 W
WBrucine, an alkaloid closely related to strychnine, is most commonly found in the Strychnos nux-vomica tree. Brucine poisoning is rare, since it is usually ingested with strychnine, and strychnine is more toxic than brucine. In synthetic chemistry, it can be used as a tool for stereospecific chemical syntheses.
 W
WCaffeine is a central nervous system (CNS) stimulant of the methylxanthine class. It is the world's most widely consumed psychoactive drug. Unlike many other psychoactive substances, it is legal and unregulated in nearly all parts of the world. There are several known mechanisms of action to explain the effects of caffeine. The most prominent is that it reversibly blocks the action of adenosine on its receptors and consequently prevents the onset of drowsiness induced by adenosine. Caffeine also stimulates certain portions of the autonomic nervous system.
 W
WCaffeine citrate, sold under the brand name Cafcit among others, is a medication used to treat a lack of breathing in premature babies. Specifically it is given to babies who are born at less than 35 weeks or weigh less than 2 kilograms (4.4 lb) once other causes are ruled out. It is given by mouth or slow injection into a vein.
 W
WDenatonium, usually available as denatonium benzoate and as denatonium saccharide (BITTERANT-s), is the most bitter chemical compound known, with bitterness thresholds of 0.05 ppm for the benzoate and 0.01 ppm for the saccharide. It was discovered in 1958 during research on local anesthetics by MacFarlan Smith of Edinburgh, Scotland, and registered under the trademark Bitrex.
 W
WEugenin is a chromone derivative, a phenolic compound found in cloves. It is also one of the compounds responsible for bitterness in carrots.
 W
WHesperidin is a flavanone glycoside found in citrus fruits. Its aglycone form is called hesperetin. Its name is derived from the word "hesperidium", for fruit produced by citrus trees.
 W
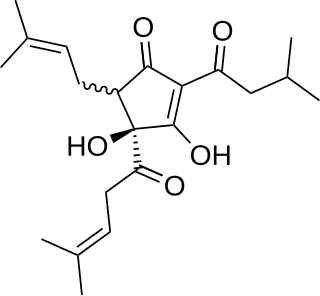
WHumulone, a vinylogous type of organic acid, is a bitter-tasting chemical compound found in the resin of mature hops. Humulone is a prevalent member of the class of compounds known as alpha acids, which collectively give hopped beer its characteristic bitter flavor.
 W
WIsohumulones are chemical compounds that contribute to the bitter taste of beer and are in the class of compounds known as iso-alpha acids. They are found in hops.
 W
WKaempferol-3-O-rutinoside is a bitter-tasting flavonol glycoside. It can be isolated from the rhizomes of the fern Selliguea feei.
 W
W6-Methoxymellein is a dihydroisocoumarin, a phenolic compound found in carrots and carrot purées. It is responsible for bitterness in carrots. It is a phytoalexin, induced in carrot slices by UV-C, that allows resistance to Botrytis cinerea and other microorganisms.
 W
WNaringin is a flavanone-7-O-glycoside between the flavanone naringenin and the disaccharide neohesperidose. The flavonoid naringin occurs naturally in citrus fruits, especially in grapefruit, where naringin is responsible for the fruit's bitter taste. In commercial grapefruit juice production, the enzyme naringinase can be used to remove the bitterness created by naringin. In humans naringin is metabolized to the aglycone naringenin by naringinase present in the gut.
 W
WPapaverine is an opium alkaloid antispasmodic drug, used primarily in the treatment of visceral spasm and vasospasm, and occasionally in the treatment of erectile dysfunction. It is used in the treatment of acute mesenteric ischemia. While it is found in the opium poppy, papaverine differs in both structure and pharmacological action from the analgesic morphine-like compounds.
 W
WPropylthiouracil (PTU) is a medication used to treat hyperthyroidism. This includes hyperthyroidism due to Graves' disease and toxic multinodular goiter. In a thyrotoxic crisis it is generally more effective than methimazole. Otherwise it is typically only used when methimazole, surgery, and radioactive iodine is not possible. It is taken by mouth.
 W
WQuassin is a white bitter, crystalline substance that is the prototypical example of the family of quassinoids. It can be extracted from the quassia tree, from which it gets its name. It was first isolated in 1937 and its chemical structure was elucidated in 1961. It is one of the most bitter substances found in nature, with a bitter threshold of 0.08 ppm and it is 50 times more bitter than quinine.
 W
WQuinidine is a medication that acts as a class I antiarrhythmic agent (Ia) in the heart. It is a stereoisomer of quinine, originally derived from the bark of the cinchona tree. The drug causes increased action potential duration, as well as a prolonged QT interval.
 W
WQuinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to Plasmodium falciparum that is resistant to chloroquine when artesunate is not available. While sometimes used for nocturnal leg cramps, quinine is not recommended for this purpose due to the risk of serious side effects. It can be taken by mouth or intravenously. Malaria resistance to quinine occurs in certain areas of the world. Quinine is also the ingredient in tonic water that gives it its bitter taste.
 W
WSalicin is an alcoholic β-glucoside. Salicin is produced in willow (Salix) bark. It is a biosynthetic precursor to salicylaldehyde.
 W
WStrychnine is a highly toxic, colorless, bitter, crystalline alkaloid used as a pesticide, particularly for killing small vertebrates such as birds and rodents. Strychnine, when inhaled, swallowed, or absorbed through the eyes or mouth, causes poisoning which results in muscular convulsions and eventually death through asphyxia. While it is no longer used medicinally, it was used historically in small doses to strengthen muscle contractions, such as a heart and bowel stimulant and performance enhancing drug. The most common source is from the seeds of the Strychnos nux-vomica tree.
 W
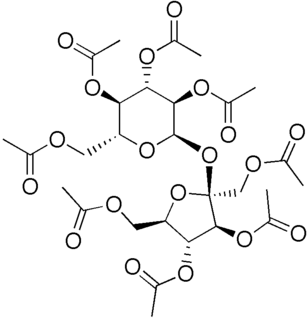
WSucrose octaacetate is a chemical compound with formula C28H38O19or (C2H3O2)8(C12H14O3), an eight-fold ester of sucrose and acetic acid. Its molecule can be described as that of sucrose C12H22O11 with its eight hydroxyl groups HO– replaced by acetate groups H3C–CO2–. It is a crystalline solid, colorless and odorless but intensely bitter.
 W
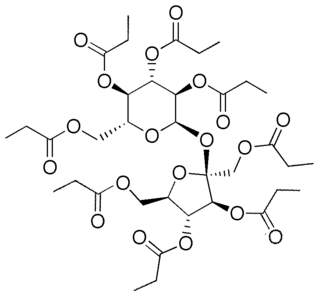
WSucrose octapropionate is a chemical compound with formula C36H54O19 or (C3H5O2)8(C12H14O3), an eight-fold ester of sucrose and propionic acid. Its molecule can be described as that of sucrose C12H22O11 with its eight hydroxyl groups HO– replaced by propionate groups H3C–CH2–CO2–. It is a crystalline colorless solid. It is also called sucrose octapropanoate or octapropionyl sucrose.
 W
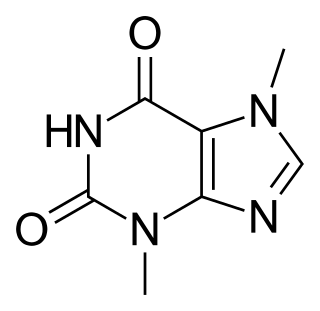
WTheobromine, also known as xantheose, is a bitter alkaloid of the cacao plant, with the chemical formula C7H8N4O2. It is found in chocolate, as well as in a number of other foods, including the leaves of the tea plant, and the kola nut. It is classified as a xanthine alkaloid (more specifically, a methylxanthine), others of which include theophylline and caffeine. Caffeine differs from these compounds in that it has an extra methyl group (see under Pharmacology section).
 W
WTheophylline, also known as 1,3-dimethylxanthine, is a phosphodiesterase inhibiting drug used in therapy for respiratory diseases such as chronic obstructive pulmonary disease (COPD) and asthma under a variety of brand names. As a member of the xanthine family, it bears structural and pharmacological similarity to theobromine and caffeine, and is readily found in nature, being present in tea and cocoa. A small amount of theophylline is one of the products of caffeine metabolic processing in the liver.