 W
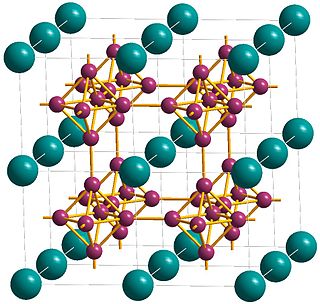
WCalcium hexaboride (sometimes calcium boride) is a compound of calcium and boron with the chemical formula CaB6. It is an important material due to its high electrical conductivity, hardness, chemical stability, and melting point. It is a black, lustrous, chemically inert powder with a low density. It has the cubic structure typical for metal hexaborides, with octahedral units of 6 boron atoms combined with calcium atoms. CaB6 and lanthanum-doped CaB6 both show weak ferromagnetic properties, which is a remarkable fact because calcium and boron are neither magnetic, nor have inner 3d or 4f electronic shells, which are usually required for ferromagnetism.
 W
WCopper(I) sulfide is a copper sulfide, a chemical compound of copper and sulfur. It has the chemical compound Cu2S. It is found in nature as the mineral chalcocite. It has a narrow range of stoichiometry ranging from Cu1.997S to Cu2.000S.
 W
WHigher sulfur oxides are a group of chemical compounds with the formula SO3+x where x lies between 0 and 1. They contain peroxo (O−O) groups, and the oxidation state of sulfur is +6 as in SO3.Monomeric SO4 can be isolated at low temperatures (below 78 K) following the reaction of SO3 and atomic oxygen or photolysis of SO3–ozone mixtures. The favoured structure is:
 W
WIron(II,III) oxide is the chemical compound with formula Fe3O4. It occurs in nature as the mineral magnetite. It is one of a number of iron oxides, the others being iron(II) oxide (FeO), which is rare, and iron(III) oxide (Fe2O3) which also occurs naturally as the mineral hematite. It contains both Fe2+ and Fe3+ ions and is sometimes formulated as FeO ∙ Fe2O3. This iron oxide is encountered in the laboratory as a black powder. It exhibits permanent magnetism and is ferrimagnetic, but is sometimes incorrectly described as ferromagnetic. Its most extensive use is as a black pigment. For this purpose, it is synthesized rather than being extracted from the naturally occurring mineral as the particle size and shape can be varied by the method of production.
 W
WIron(II) oxide or ferrous oxide is the inorganic compound with the formula FeO. Its mineral form is known as wüstite. One of several iron oxides, it is a black-colored powder that is sometimes confused with rust, the latter of which consists of hydrated iron(III) oxide. Iron(II) oxide also refers to a family of related non-stoichiometric compounds, which are typically iron deficient with compositions ranging from Fe0.84O to Fe0.95O.
 W
WIron(II) sulfide or ferrous sulfide is one of a family chemical compounds and minerals with the approximate formula FeS. Iron sulfides are often iron-deficient non-stoichiometric. All are black, water-insoluble solids.
 W
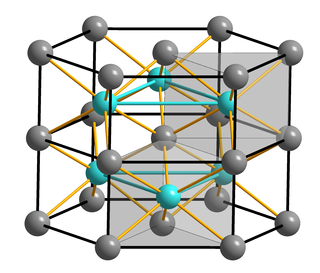
WMagnesium diboride is the inorganic compound with the formula MgB2. It is a dark gray, water-insoluble solid. The compound has attracted attention because it becomes superconducting at 39 K (−234 °C). In terms of its composition, MgB2 differs strikingly from most low-temperature superconductors, which feature mainly transition metals. Its superconducting mechanism is primarily described by BCS theory.
 W
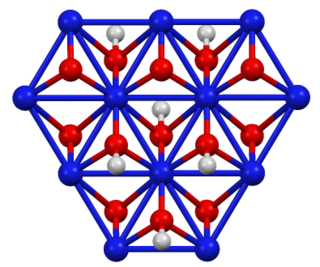
WNickel oxide hydroxide is the inorganic compound with the chemical formula NiO(OH). It is a black solid that is insoluble in all solvents but attacked by base and acid. It is a component of the nickel-metal hydride battery and of the nickel–iron battery.
 W
WNickel(II) oxide is the chemical compound with the formula NiO. It is the principal oxide of nickel. It is classified as a basic metal oxide. Several million kilograms are produced annually of varying quality, mainly as an intermediate in the production of nickel alloys. The mineralogical form of NiO, bunsenite, is very rare. Other nickel(III) oxides have been claimed, for example: Ni2O3 and NiO2, but they have yet to be proven by X-ray crystallography.
 W
WNickel(III) oxide is the inorganic compound with the formula Ni2O3. It is not well characterized, and is sometimes referred to as black nickel oxide. Traces of Ni2O3 on nickel surfaces have been mentioned.
 W
WNiobium dioxide, is the chemical compound with the formula NbO2. It is a bluish-black non-stoichiometric solid with a composition range of NbO1.94-NbO2.09. It can be prepared by reducing Nb2O5 with H2 at 800–1350 °C. An alternative method is reaction of Nb2O5 with Nb powder at 1100 °C.
 W
WNon-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having elemental composition whose proportions cannot be represented by a ratio of small natural numbers; most often, in such materials, some small percentage of atoms are missing or too many atoms are packed into an otherwise perfect lattice work.
 W
WSilver telluride (Ag2Te) is a chemical compound, a telluride of silver, also known as disilver telluride or silver(I) telluride. It forms a monoclinic crystal. In a wider sense, silver telluride can be used to denote AgTe (silver(II) telluride, a metastable compound) or Ag5Te3.
 W
WSodium tungsten bronze is a form of insertion compound with the formula NaxWO3, where x is equal to or less than 1. So named because of its metallic lustre, its electrical properties range from semiconducting to metallic depending on the concentration of sodium ions present; it can also exhibit superconductivity.
 W
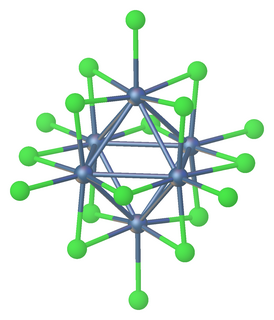
WTantalum(III) chloride or tantalum trichloride is non-stoichiometric with a range of composition from TaCl2.9 to TaCl3.1 Anionic and neutral clusters containing Ta(III) chloride include [Ta6Cl18]4− and [Ta6Cl14](H2O)4.
 W
WTitanium(II) oxide (TiO) is an inorganic chemical compound of titanium and oxygen. It can be prepared from titanium dioxide and titanium metal at 1500 °C. It is non-stoichiometric in a range TiO0.7 to TiO1.3 and this is caused by vacancies of either Ti or O in the defect rock salt structure. In pure TiO 15% of both Ti and O sites are vacant. Careful annealing can cause ordering of the vacancies producing a monoclinic form which has 5 TiO units in the primitive cell that exhibits lower resistivity. A high temperature form with titanium atoms with trigonal prismatic coordination is also known. Acid solutions of TiO are stable for a short time then decompose to give hydrogen:2 Ti2+(aq) + 2 H+(aq) → 2 Ti3+(aq) + H2(g)
 W
WVanadium(II) oxide, VO, is one of the many oxides of vanadium. VO is a long-lived, electronically neutral reagent chemical. It adopts a distorted NaCl structure and contains weak V−V metal to metal bonds. As shown by band theory, VO is a conductor of electricity due to its partially filled conduction band and delocalisation of electrons in the t2g orbitals. VO is a non-stoichiometric compound, its composition varying from VO0.8 to VO1.3. Gaseous VO is one of the molecules found in the spectrum of relatively cool M-type stars.
 W
WWüstite (FeO) is a mineral form of iron(II) oxide found with meteorites and native iron. It has a grey colour with a greenish tint in reflected light. Wüstite crystallizes in the isometric-hexoctahedral crystal system in opaque to translucent metallic grains. It has a Mohs hardness of 5 to 5.5 and a specific gravity of 5.88. Wüstite is a typical example of a non-stoichiometric compound.
 W
WYttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds, famous for displaying high-temperature superconductivity. It includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen (77 K) at about 92 K. Many YBCO compounds have the general formula YBa2Cu3O7−x (also known as Y123), although materials with other Y:Ba:Cu ratios exist, such as YBa2Cu4Oy (Y124) or Y2Ba4Cu7Oy (Y247). At present, there is no singularly recognised theory for high-temperature superconductivity.