 W
WAA560 is an orally active nonsteroidal antiandrogen (NSAA) that was developed in Japan and was first described in the literature in 1977 but was never marketed. It is an anilide derivative and analogue of the NSAA flutamide, and shows greater in vivo antiandrogenic potency than does flutamide. Similarly to flutamide, AA560 is a selective antagonist of the androgen receptor (AR) and consequently shows progonadotropic effects by increasing levels of gonadotropins and testosterone via disinhibition of the hypothalamic-pituitary-gonadal axis.
 W
WAmphenone B, or simply amphenone, also known as 3,3-bis(p-aminophenyl)butan-2-one, is an inhibitor of steroid hormone and thyroid hormone biosynthesis which was never marketed but has been used as a tool in scientific research to study corticosteroids and the adrenal glands. It acts as competitive inhibitor of 11β-hydroxylase, 17α-hydroxylase, 17,20-lyase, 21-hydroxylase, and 3β-hydroxysteroid dehydrogenase, as well as of cholesterol side-chain cleavage enzyme, thereby inhibiting the production of steroid hormones including glucocorticoids, mineralocorticoids, androgens, and estrogens. In addition, amphenone B inhibits the production of thyroxine by a thiouracil-like mechanism, specifically via inhibition of organic binding of iodine and uptake of iodide by the thyroid gland.
 W
WApalutamide, sold under the brand name Erleada among others, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is specifically indicated for use in conjunction with castration in the treatment of non-metastatic castration-resistant prostate cancer (NM-CRPC). It is taken by mouth.
 W
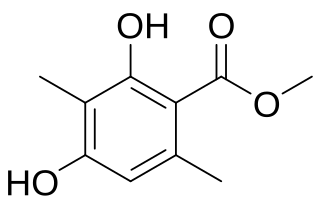
WAtraric acid is a naturally occurring phenolic compound and ester with the IUPAC name methyl 2,4-dihydroxy-3,6-dimethylbenzoate and molecular formula C10H12O4. It occurs in the root-bark of Pygeum africanum and Evernia prunastri (Oakmoss). There is evidence to suggest that it has antiandrogenic activity in humans and its use in treatment of benign prostate hyperplasia, prostate cancer, and spinal and bulbar muscular atrophy has been investigated.
 W
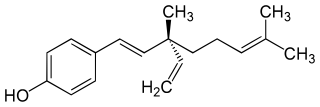
WBakuchiol is a meroterpene in the class terpenophenol.
 W
WBicalutamide, sold under the brand name Casodex among others, is an antiandrogen medication that is primarily used to treat prostate cancer. It is typically used together with a gonadotropin-releasing hormone (GnRH) analogue or surgical removal of the testicles to treat advanced prostate cancer. Bicalutamide may also be used to treat excessive hair growth in women, as a component of feminizing hormone therapy for transgender women, to treat early puberty in boys, and to prevent overly long-lasting erections in men. It is taken by mouth.
 W
W5N-Bicalutamide, or 5-azabicalutamide, is a highly potent nonsteroidal antiandrogen (NSAA) which was discovered in 2016. It is a structural modification of bicalutamide differing it from it only by the replacement of a carbon atom with a nitrogen atom in one of its phenyl rings. Similarly to bicalutamide, the drug acts as a selective antagonist of the androgen receptor (AR). However, unlike bicalutamide, it is a reversible covalent antagonist and stays bound to the receptor for a far longer amount of time. As a result of this difference, 5N-bicalutamide has markedly improved potency relative to bicalutamide, with approximately 150-fold higher affinity for the AR (Ki = 0.15 nM versus 22.3 nM) and about 20-fold greater functional inhibition (IC50 = 15 nM versus 310 nM) of the AR. Future studies of 5N-bicalutamide in normal and mutated prostate cancer cells are planned or underway and it is anticipated that N-bicalutamide may be able to overcome resistance to current antiandrogens that are used in the treatment of prostate cancer.
 W
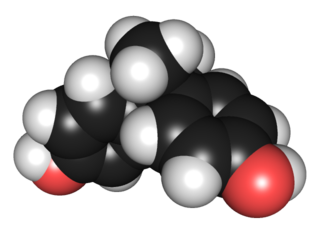
WBisphenol A (BPA) is an organic synthetic compound with the chemical formula (CH3)2C(C6H4OH)2 belonging to the group of diphenylmethane derivatives and bisphenols, with two hydroxyphenyl groups. It is a colorless solid that is soluble in organic solvents, but poorly soluble in water (0.344 wt % at 83 °C).
 W
WBisphenol A diglycidyl ether is an organic compound used as constituent of epoxy resins. The compound is a colorless solid that melts slightly above room temperature.
 W
WBisphenol S (BPS) is an organic compound with the formula (HOC6H4)2SO2. It has two phenol functional groups on either side of a sulfonyl group. It is commonly used in curing fast-drying epoxy resin adhesives. It is a bisphenol, and a close analog of bisphenol A (BPA) in which the dimethylmethylene group (C(CH3)2) is replaced with a sulfone group (SO2).
 W
WBMS-641988 is a nonsteroidal antiandrogen which was developed by Bristol-Myers Squibb for the treatment of prostate cancer but was never marketed. It acts as a potent competitive antagonist of the androgen receptor (AR) (Ki = 10 nM; IC50 = 56 nM). The drug was found to have 20-fold higher affinity for the AR than bicalutamide in MDA-MB-453 cells, and showed 3- to 7-fold the antiandrogenic activity of bicalutamide in vitro. It may have some weak partial agonist activity at the androgen receptor. BMS-641988 is transformed by CYP3A4 into BMS-570511, and this metabolite is then reduced to BMS-501949 by cytosolic reductases. All three compounds show similar antiandrogenic activity. In addition to its antiandrogenic activity, BMS-641988 shows activity as a negative allosteric modulator of the GABAA receptor, and can produce seizures in animals at sufficiently high doses. It also shows some drug-induced QT prolongation. BMS-641988 reached phase I clinical trials prior to the discontinuation of its development. The clinical development of BMS-641988 was terminated due to the occurrence of a seizure in a patient during a phase I study.
 W
WCimetidine, sold under the brand name Tagamet among others, is a histamine H2 receptor antagonist that inhibits stomach acid production. It is mainly used in the treatment of heartburn and peptic ulcers.
 W
WCioteronel is a nonsteroidal antiandrogen (NSAA) that was never marketed. It was under development between 1989 and 2001 for the topical treatment of androgenetic alopecia and acne and for the oral treatment of benign prostatic hyperplasia; it reached phase III clinical trials for acne and phase II studies for androgenetic alopecia, but was ultimately discontinued due to poor efficacy.
 W
WCyanonilutamide is a nonsteroidal antiandrogen which was never marketed. Both RU-56187 and RU-58841 appear to be prodrugs of cyanonilutamide in vivo in animals. It has relatively low affinity for the androgen receptor but nonetheless shows significant antiandrogenic activity in animals.
 W
WDichlorodiphenyltrichloroethane, commonly known as DDT, is a colorless, tasteless, and almost odorless crystalline chemical compound, an organochlorine. Originally developed as an insecticide, it became infamous for its environmental impacts. DDT was first synthesized in 1874 by the Austrian chemist Othmar Zeidler. DDT's insecticidal action was discovered by the Swiss chemist Paul Hermann Müller in 1939. DDT was used in the second half of World War II to limit the spread of the insect-born diseases malaria and typhus among civilians and troops. Müller was awarded the Nobel Prize in Physiology or Medicine in 1948 "for his discovery of the high efficiency of DDT as a contact poison against several arthropods".
 W
WN-Desmethylapalutamide is a nonsteroidal antiandrogen (NSAA) and the major active metabolite of apalutamide, an NSAA which is used as a hormonal antineoplastic agent in the treatment of metastatic prostate cancer. It has similar activity to that of apalutamide and, with apalutamide therapy, circulates at similar concentrations to those of apalutamide at steady state. N-Desmethylapalutamide is formed from apalutamide in the liver by the cytochrome P450 enzymes CYP2C8 and CYP3A4.
 W
WN-Desmethylenzalutamide is a nonsteroidal antiandrogen (NSAA) and the major metabolite of enzalutamide, an NSAA which is used as a hormonal antineoplastic agent in the treatment of metastatic prostate cancer. It has similar activity to that of enzalutamide and, with enzalutamide therapy, circulates at similar concentrations to those of enzalutamide at steady state. N-Desmethylenzalutamide is formed from enzalutamide in the liver by the cytochrome P450 enzymes CYP2C8 and CYP3A4. It has a longer terminal half-life than enzalutamide.
 W
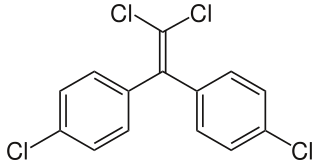
WDichlorodiphenyldichloroethylene (DDE) is a chemical compound formed by the loss of hydrogen chloride (dehydrohalogenation) from DDT, of which it is one of the more common breakdown products. Due to DDT’s massive prevalence in society and agriculture during the mid 20th century, DDT and DDE are still widely seen in animal tissue samples. DDE is particularly dangerous because it is fat-soluble like other organochlorines; thus, it is rarely excreted from the body, and concentrations tend to increase throughout life. The major exception is the excretion of DDE in breast milk, which transfers a substantial portion of the mother's DDE burden to the young animal or child. Along with accumulation over an organism's lifetime, this stability leads to bioaccumulation in the environment, which amplifies DDE’s negative effects.
 W
WDieldrin is an organochloride originally produced in 1948 by J. Hyman & Co, Denver, as an insecticide. Dieldrin is closely related to aldrin, which reacts further to form dieldrin. Aldrin is not toxic to insects; it is oxidized in the insect to form dieldrin which is the active compound. Both dieldrin and aldrin are named after the Diels-Alder reaction which is used to form aldrin from a mixture of norbornadiene and hexachlorocyclopentadiene.
 W
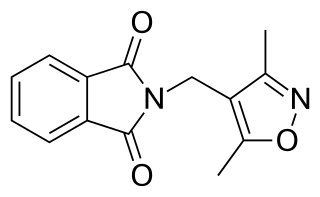
WDIMP, or N-(3,5-dimethyl-4-isoxazolylmethyl)phthalimide, is a nonsteroidal antiandrogen (NSAA) structurally related to thalidomide that was first described in 1973 and was never marketed. Along with flutamide, it was one of the earliest NSAAs to be discovered, and for this reason, has been described as a "classical" NSAA. The drug is a selective, competitive, silent antagonist of the AR, although it is described as an "only relatively weak competitor". Its relative binding affinity for the androgen receptor is about 2.6% of that of metribolone. DIMP possesses no androgenic, estrogenic, progestogenic, or antigonadotropic activity, but it does reverse the antigonadotropic effects of testosterone, indicating that, like other pure AR antagonists, it is progonadotropic.
 W
WEndosulfan is an off-patent organochlorine insecticide and acaricide that is being phased out globally. The two isomers, endo and exo, are known popularly as I and II. Endosulfan sulfate is a product of oxidation containing one extra O atom attached to the S atom. Endosulfan became a highly controversial agrichemical due to its acute toxicity, potential for bioaccumulation, and role as an endocrine disruptor. Because of its threats to human health and the environment, a global ban on the manufacture and use of endosulfan was negotiated under the Stockholm Convention in April 2011. The ban has taken effect in mid-2012, with certain uses exempted for five additional years. More than 80 countries, including the European Union, Australia, New Zealand, several West African nations, the United States, Brazil, and Canada had already banned it or announced phase-outs by the time the Stockholm Convention ban was agreed upon. It is still used extensively in India, China despite laws banning it, and few other countries. It is produced by Makhteshim Agan and several manufacturers in India and China. Although, the Supreme Court had, by an order dated 13.05.2011, put a ban on the production and sale of endosulfan in India till further orders.
 W
WEnzalutamide, sold under the brand name Xtandi, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is indicated for use in conjunction with castration in the treatment of metastatic castration-resistant prostate cancer (mCRPC), nonmetastatic castration-resistant prostate cancer, and metastatic castration-sensitive prostate cancer (mCSPC). It is taken by mouth.
 W
WFenarimol, sold under the tradenames Bloc, Rimidin and Rubigan, is a fungicide which acts against rusts, blackspot and mildew fungi. It is used on ornamental plants, trees, lawns, tomatoes, peppers, eggplants, cucumbers and melons. It is mainly used to control powdery mildew. It works by inhibiting the fungus's biosynthesis of important steroid molecules.
 W
WFlutamide, sold under the brand name Eulexin among others, is a nonsteroidal antiandrogen (NSAA) which is used primarily to treat prostate cancer. It is also used in the treatment of androgen-dependent conditions like acne, excessive hair growth, and high androgen levels in women. It is taken by mouth, usually three times per day.
 W
WBisphenol A controversy centers on concerns and debates about the biomedical significance of bisphenol A (BPA), which is a precursor to polymers that are used in some consumer products, including some food containers. The concerns began with the hypothesis that BPA is an endocrine disruptor, i.e. it mimics endocrine hormones and thus has the unintended and possibly far-reaching effects on people in physical contact with the chemical.
 W
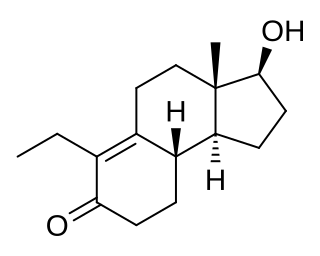
WHydroxyflutamide (HF, OHF) (developmental code name SCH-16423), or 2-hydroxyflutamide, is a nonsteroidal antiandrogen (NSAA) and the major active metabolite of flutamide, which is considered to be a prodrug of hydroxyflutamide as the active form. It has been reported to possess an IC50 of 700 nM for the androgen receptor (AR), which is about 4-fold less than that of bicalutamide.
 W
WInocoterone is a steroid-like nonsteroidal antiandrogen (NSAA) that was never marketed. An acetate ester, inocoterone acetate, shows greater antiandrogen activity and was developed as a topical medication for the treatment of acne but showed only modest effectiveness in clinical trials and similarly was never marketed.
 W
WInocoterone acetate (USAN) is a steroid-like nonsteroidal antiandrogen (NSAA) that was developed for topical administration to treat acne but was never marketed. It is the acetate ester of inocoterone, which is less potent in comparison. Inocoterone acetate is actually not a silent antagonist of the androgen receptor but rather a weak partial agonist, similarly to steroidal antiandrogens like cyproterone acetate.
 W
WKetoconazole, sold under the brand name Nizoral among others, is an antifungal medication used to treat a number of fungal infections. Applied to the skin it is used for fungal skin infections such as tinea, cutaneous candidiasis, pityriasis versicolor, dandruff, and seborrheic dermatitis. Taken by mouth it is a less preferred option and only recommended for severe infections when other agents cannot be used. Other uses include in the treatment of excessive hair growth and Cushing's syndrome.
 W
WKetodarolutamide is a nonsteroidal antiandrogen (NSAA) and the major active metabolite of darolutamide, an NSAA which is used in the treatment of prostate cancer in men. Similarly to its parent compound, darolutamide acts as a highly selective, high-affinity, competitive silent antagonist of the androgen receptor (AR). Both agents show much higher affinity and more potent inhibition of the AR relative to the other NSAAs enzalutamide and apalutamide, although they also possess much shorter and comparatively less favorable elimination half-lives. They have also been found not to activate certain mutant AR variants that enzalutamide and apalutamide do activate. Both darolutamide and ketodarolutamide show limited central nervous system distribution, indicating peripheral selectivity, and little or no inhibition or induction of cytochrome P450 enzymes such as CYP3A4, unlike enzalutamide and apalutamide.
 W
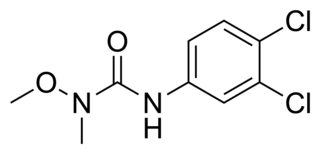
WLG-120907 is a nonsteroidal antiandrogen (NSAA) of the quinoline group which was developed by Ligand Pharmaceuticals along with selective androgen receptor modulators (SARMs) like LG-121071 and was never marketed. The drug is a high-affinity antagonist of the androgen receptor (AR) with a Ki value of 26 nM and has been found to inhibit growth of the ventral prostate and seminal vesicles in male rats without increasing circulating levels of luteinizing hormone or testosterone. However, this tissue selectivity has not been assessed in humans. LG-120907 is orally active and shows greater oral potency than the arylpropionamide NSAA flutamide.
 W
WLinuron is a phenylurea herbicide that is used to control the growth of grass and weeds for the purpose of supporting the growth of crops like soybeans.
 W
WMethiocarb is a carbamate pesticide which is used as a bird repellent, insecticide, acaricide and molluscicide since the 1960s. Carbamates are widely used in agriculture as insecticides and herbicides. They are preferred instead of organochlorines because organochlorines are long lasting persistent in crops. Methiocarb has contact and stomach action on mites and neurotoxic effects on molluscs. Seeds treated with methiocarb also affects birds. Other names for methiocarb are mesurol and mercaptodimethur.
 W
WNilutamide, sold under the brand names Nilandron and Anandron, is a nonsteroidal antiandrogen (NSAA) which is used in the treatment of prostate cancer. It has also been studied as a component of feminizing hormone therapy for transgender women and to treat acne and seborrhea in women. It is taken by mouth.
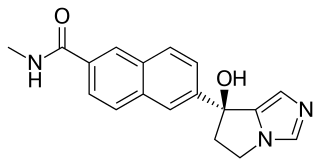 W
WOrteronel (TAK-700) is a nonsteroidal CYP17A1 inhibitor that was being developed for the treatment of cancer by Takeda Pharmaceutical Company in conjunction with Millennium Pharmaceuticals. It completed two phase III clinical trials for metastatic, hormone-refractory prostate cancer but failed to extend overall survival rates, and development was voluntarily terminated as a result.
 W
WPentomone is a nonsteroidal antiandrogen (NSAA) described as a "prostate growth inhibitor" which was never marketed. It was synthesized and assayed in 1978.
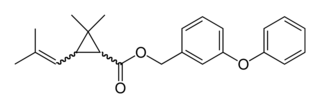 W
WPhenothrin, also called sumithrin and d-phenothrin, is a synthetic pyrethroid that kills adult fleas and ticks. It has also been used to kill head lice in humans. d-Phenothrin is used as a component of aerosol insecticides for domestic use. It is often used with methoprene, an insect growth regulator that interrupts the insect's biological lifecycle by killing the eggs. Phenothrin is the active agent in the branded product Raid Fly and Wasp Killer.
 W
WProchloraz, brand name Sportak, is an imidazole fungicide that was introduced in 1978 and is widely used in Europe, Australia, Asia, and South America within gardening and agriculture to control the growth of fungi. It is not registered for use in the United States. Similarly to other azole fungicides, prochloraz is an inhibitor of the enzyme lanosterol 14α-demethylase (CYP51A1), which is necessary for the production of ergosterol – an essential component of the fungal cell membrane – from lanosterol. The agent is a broad-spectrum, protective and curative fungicide, effective against Alternaria spp., Botrytis spp., Erysiphe spp., Helminthosporium spp., Fusarium spp., Pseudocerosporella spp., Pyrenophora spp., Rhynchosporium spp., and Septoria spp.
 W
WProcymidone is a pesticide. It is often used for killing unwanted ferns and nettles, and as a dicarboximide fungicide for killing fungi, for example as seed dressing, pre-harvest spray or post-harvest dip of lupins, grapes, stone fruit, strawberries. It is a known endocrine disruptor which interferes with the sexual differention of male rats. It is considered to be a poison.
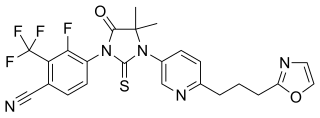 W
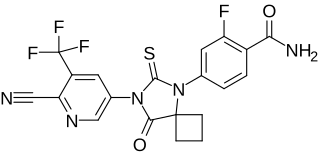
WProxalutamide is a nonsteroidal antiandrogen (NSAA) – specifically, a selective high-affinity silent antagonist of the androgen receptor (AR) – which is under development by Suzhou Kintor Pharmaceuticals for the treatment of prostate cancer. It inhibits AR-mediated gene transcription more potently than bicalutamide and enzalutamide and maintains silent antagonism in castration-resistant prostate cancer (CRPC) cells. It has also been found to downregulate the AR, which could further confer it greater efficacy against CRPC compared to existing NSAAs. Unlike enzalutamide, the drug showed low central nervous system distribution and no induction of seizures in animals. As of 2017, it is in phase II clinical trials for prostate cancer. It is also in preclinical investigation for the treatment of AR-positive breast cancer.
 W
WRalaniten acetate is a first-in-class antiandrogen that targets the N-terminal domain (NTD) of the androgen receptor (AR) developed by ESSA Pharmaceuticals and was under investigation for the treatment of prostate cancer. This mechanism of action is believed to allow the drug to block signaling from the AR and its splice variants. EPI-506 is a derivative of bisphenol A and a prodrug of ralaniten (EPI-002), one of the four stereoisomers of EPI-001, and was developed as a successor of EPI-001. The drug reached phase I/II prior to the discontinuation of its development. It showed signs of efficacy in the form of prostatic specific antigen (PSA) decreases (4–29%) predominantly at higher doses (≥1,280 mg) in some patients but also caused side effects and was discontinued by its developer in favor of next-generation AR NTD inhibitors with improved potency and tolerability.
 W
WRD-162 is a second-generation nonsteroidal antiandrogen (NSAA) which was developed for the treatment of prostate cancer but was never marketed. It acts as a potent and selective silent antagonist of the androgen receptor (AR). The drug is a diarylthiohydantoin derivative. It is closely related to enzalutamide and apalutamide. Both RD-162 and enzalutamide show 5- to 8-fold higher affinity for the AR than the first-generation NSAA bicalutamide, and only 2- to 3-fold lower affinity than dihydrotestosterone (DHT), the major endogenous ligand of the receptor in the prostate gland.
 W
WRezvilutamide is a nonsteroidal antiandrogen which is under development by Jiangsu Hengrui Medicine for the treatment of prostate cancer and breast cancer. It is a selective androgen receptor antagonist with reduced brain distribution compared to the structurally related nonsteroidal antiandrogen enzalutamide. As of October 2020, rezvilutamide is in phase 3 clinical trials for prostate cancer. Other structural analogues of rezvilutamide besides enzalutamide include apalutamide and proxalutamide.
 W
WRU-22930 is a nonsteroidal antiandrogen (NSAA) related to the NSAAs flutamide and nilutamide (RU-23908) and was developed by Roussel Uclaf but was never marketed. It is a selective antagonist of the androgen receptor and consequently has progonadotropic effects by increasing gonadotropin and testosterone levels via disinhibition of the hypothalamic-pituitary-gonadal axis. Unlike flutamide and nilutamide, the drug is said to be short-acting and inactive by injection, but it has been found to be active topically in animals, and hence could be useful for the treatment of androgen-dependent skin conditions.
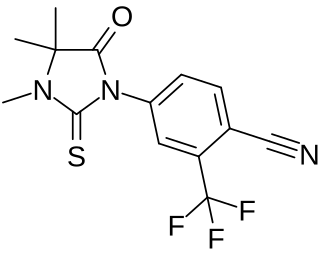 W
WRU-56187 is a nonsteroidal antiandrogen which was never marketed. It shows 92% of the affinity of testosterone for the androgen receptor and negligible affinity for other steroid hormone receptors. The medication is a silent antagonist of the androgen receptor. RU-56187 is 3- to 10-fold more potent as an antiandrogen than bicalutamide or nilutamide in animals. Both RU-56187 and RU-58841 appear to be prodrugs of cyanonilutamide (RU-56279) in vivo in animals.
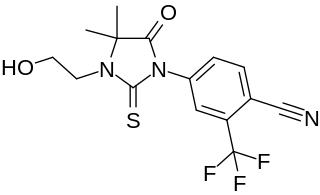 W
WRU-57073 is a nonsteroidal antiandrogen which was never marketed. It shows 163% of the affinity of testosterone for the androgen receptor and negligible affinity for other steroid hormone receptors.
 W
WRU-58642 is a nonsteroidal antiandrogen (NSAA) derived from nilutamide with very high affinity and selectivity for the androgen receptor (AR), which made it among the most potent and efficacious antiandrogens known at the time of its discovery. It was investigated for topical application for the treatment of androgenetic alopecia, but development did not proceed past initial trial stages, and it is now only used for scientific research into the AR.
 W
WRU-58841, also known as PSK-3841 or HMR-3841, is a nonsteroidal antiandrogen (NSAA) which was initially developed in the 1980s. It was formerly under investigation by ProStrakan for potential use as a topical treatment for androgen-dependent conditions including acne, pattern hair loss, and excessive hair growth. The compound is similar in structure to the NSAA RU-58642 but contains a different side-chain. These compounds are similar in chemical structure to nilutamide, which is related to flutamide, bicalutamide, and enzalutamide, all of which are NSAAs similarly. RU-58841 can be synthesized either by building the hydantoin moiety or by aryl coupling to 5,5-dimethylhydantoin.
 W
WSeviteronel is an experimental cancer medication which is under development by Viamet Pharmaceuticals and Innocrin Pharmaceuticals for the treatment of prostate cancer and breast cancer. It is a nonsteroidal CYP17A1 inhibitor and works by inhibiting the production of androgens and estrogens in the body. As of July 2017, seviteronel is in phase II clinical trials for both prostate cancer and breast cancer. In January 2016, it was designated fast-track status by the United States Food and Drug Administration for prostate cancer. In April 2017, seviteronel received fast-track designation for breast cancer as well.
 W
WThalidomide, sold under the brand name Thalomid among others, is a medication used to treat a number of cancers including multiple myeloma, graft-versus-host disease, and a number of skin conditions including complications of leprosy. While it has been used in a number of HIV associated conditions, such use is associated with increased levels of the virus. It is administered orally.
 W
WIn the late 1950s and early 1960s, the use of thalidomide in pregnant women in 46 countries resulted in the "biggest man‐made medical disaster ever", resulting in more than 10,000 children born with a range of severe deformities, such as phocomelia, as well as thousands of miscarriages.
 W
WTopilutamide, known more commonly as fluridil and sold under the brand name Eucapil, is an antiandrogen medication which is used in the treatment of pattern hair loss in men and women. It is used as a topical medication and is applied to the scalp.
 W
WValproate (VPA) and its valproic acid, sodium valproate, and valproate semisodium forms are medications primarily used to treat epilepsy and bipolar disorder and prevent migraine headaches. They are useful for the prevention of seizures in those with absence seizures, partial seizures, and generalized seizures. They can be given intravenously or by mouth, and the tablet forms exist in both long- and short-acting formulations.
 W
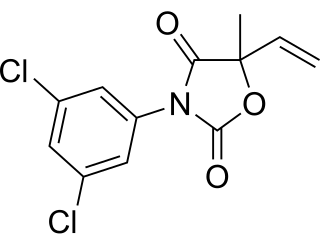
WVinclozolin is a common dicarboximide fungicide used to control diseases, such as blights, rots and molds in vineyards, and on fruits and vegetables such as raspberries, lettuce, kiwi, snap beans, and onions. It is also used on turf on golf courses. Two common fungi that vinclozolin is used to protect crops against are Botrytis cinerea and Sclerotinia sclerotiorum. First registered in 1981, vinclozolin is widely used but its overall application has declined. As a pesticide, vinclozolin is regulated by the United States Environmental Protection Agency. In addition to these restrictions within the United States, as of 2006 the use of this pesticide was banned in several countries, including Denmark, Finland, Norway, and Sweden. It has gone through a series of tests and regulations in order to evaluate the risks and hazards to the environment and animals. Among the research, a main finding is that vinclozolin has been shown to be an endocrine disruptor with antiandrogenic effects.