 W
WCrown ethers are cyclic chemical compounds that consist of a ring containing several ether groups. The most common crown ethers are cyclic oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., –CH2CH2O–. Important members of this series are the tetramer (n = 4), the pentamer (n = 5), and the hexamer (n = 6). The term "crown" refers to the resemblance between the structure of a crown ether bound to a cation, and a crown sitting on a person's head. The first number in a crown ether's name refers to the number of atoms in the cycle, and the second number refers to the number of those atoms that are oxygen. Crown ethers are much broader than the oligomers of ethylene oxide; an important group are derived from catechol.
 W
W9-Crown-3, also called 1,4,7-trioxonane or 1,4,7-trioxacyclononane is a crown ether with the formula C6H12O3. It is a cyclic trimer of ethylene oxide which is specific for the lithium cation.
 W
W12-Crown-4, also called 1,4,7,10-tetraoxacyclododecane and lithium ionophore V, is a crown ether with the formula C8H16O4. It is a cyclic tetramer of ethylene oxide which is specific for the lithium cation.
 W
W15-Crown-5 is a crown ether with the formula (C2H4O)5. It is a cyclic pentamer of ethylene oxide that forms complex with various cations, including sodium (Na+) and potassium (K+), however, it is complementary to Na+ and thus has a higher selectivity for Na+ ions.
 W
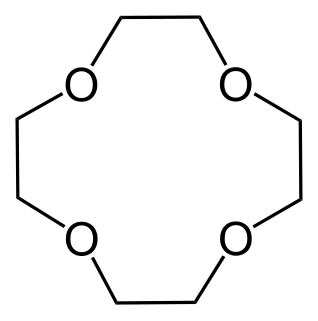
W18-Crown-6 is an organic compound with the formula [C2H4O]6 and the IUPAC name of 1,4,7,10,13,16-hexaoxacyclooctadecane. It is a white, hygroscopic crystalline solid with a low melting point. Like other crown ethers, 18-crown-6 functions as a ligand for some metal cations with a particular affinity for potassium cations (binding constant in methanol: 106 M−1). The point group of 18-crown-6 is S6. The dipole moment of 18-crown-6 varies in different solvent and under different temperature. Under 25 °C, the dipole moment of 18-crown-6 is 2.76 ± 0.06 D in cyclohexane and 2.73 ± 0.02 in benzene. The synthesis of the crown ethers led to the awarding of the Nobel Prize in Chemistry to Charles J. Pedersen.
 W
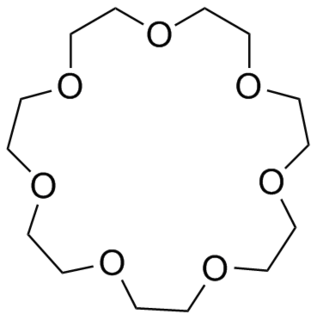
W21-Crown-7 is an organic compound with the formula [C2H4O]7 and the IUPAC name of 1,4,7,10,13,16,19-heptaoxacycloheneicosane. Like other crown ethers, 21-crown-7 functions as a ligand for some metal cations with a particular affinity for caesium cations. The dipole moment of 21-crown-7 varies in different solvents and under different temperatures.
 W
WDibenzo-18-crown-6 is the organic compound with the formula [OC6H4OCH2CH2OCH2CH2]2. It is a white solid that is soluble in organic solvents. As one of the most popular crown ethers, it facilitates the dissolution of many salts in organic solvents. It is related to the non-benzannulated 18-crown-6.
 W
W1,4-Dioxane is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers are rarely encountered.
 W
WMetallacrowns are a unique class of macrocyclic compounds that consist of metal ions and solely or predominantly heteroatoms in the ring. Classically, metallacrowns contain an [M–N–O] repeat unit in the macrocycle. First discovered by Vincent L. Pecoraro and Myoung Soo Lah in 1989, metallacrowns are best described as inorganic analogues of crown ethers. To date, over 600 reports of metallacrown research have been published. Metallacrowns with sizes ranging from 12-MC-4 to 60-MC-20 have been synthesized.