 W
WDiffusion is the net movement of anything from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in concentration.
 W
WAtomic diffusion is a diffusion process whereby the random thermally-activated movement of atoms in a solid results in the net transport of atoms. For example, helium atoms inside a balloon can diffuse through the wall of the balloon and escape, resulting in the balloon slowly deflating. Other air molecules have lower mobilities and thus diffuse more slowly through the balloon wall. There is a concentration gradient in the balloon wall, because the balloon was initially filled with helium, and thus there is plenty of helium on the inside, but there is relatively little helium on the outside. The rate of transport is governed by the diffusivity and the concentration gradient.
 W
WDialysis tubing, also known as Visking tubing, is an artificial semi-permeable membrane tubing used in separation techniques, that facilitates the flow of tiny molecules in solution based on differential diffusion. In the context of life science research, dialysis tubing is typically used in the sample clean-up and processing of proteins and DNA samples or complex biological samples such as blood or serums. Dialysis tubing is also frequently used as a teaching aid to demonstrate the principles of diffusion, osmosis, Brownian motion and the movement of molecules across a restrictive membrane. For the principles and usage of dialysis in a research setting, see Dialysis (biochemistry).
 W
WDiffusion of innovations is a theory that seeks to explain how, why, and at what rate new ideas and technology spread. Everett Rogers, a professor of communication studies, popularized the theory in his book Diffusion of Innovations; the book was first published in 1962, and is now in its fifth edition (2003). Rogers argues that diffusion is the process by which an innovation is communicated over time among the participants in a social system. The origins of the diffusion of innovations theory are varied and span multiple disciplines.
 W
WFacilitated diffusion is the process of spontaneous passive transport of molecules or ions across a biological membrane via specific transmembrane integral proteins. Being passive, facilitated transport does not directly require chemical energy from ATP hydrolysis in the transport step itself; rather, molecules and ions move down their concentration gradient reflecting its diffusive nature.
 W
WFick's laws of diffusion describe diffusion and were derived by Adolf Fick in 1855. They can be used to solve for the diffusion coefficient, D. Fick's first law can be used to derive his second law which in turn is identical to the diffusion equation.
 W
WForward osmosis (FO) is an osmotic process that, like reverse osmosis (RO), uses a semi-permeable membrane to effect separation of water from dissolved solutes. The driving force for this separation is an osmotic pressure gradient, such that a "draw" solution of high concentration, is used to induce a net flow of water through the membrane into the draw solution, thus effectively separating the feed water from its solutes. In contrast, the reverse osmosis process uses hydraulic pressure as the driving force for separation, which serves to counteract the osmotic pressure gradient that would otherwise favor water flux from the permeate to the feed. Hence significantly more energy is required for reverse osmosis compared to forward osmosis.
 W
WIn mathematics and physics, the heat equation is a certain partial differential equation. Solutions of the heat equation are sometimes known as caloric functions. The theory of the heat equation was first developed by Joseph Fourier in 1822 for the purpose of modeling how a quantity such as heat diffuses through a given region.
 W
WThe hype cycle is a branded graphical presentation developed and used by the American research, advisory and information technology firm Gartner to represent the maturity, adoption, and social application of specific technologies. The hype cycle claims to provide a graphical and conceptual presentation of the maturity of emerging technologies through five phases.
 W
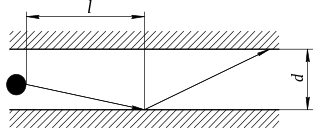
WIn physics, Knudsen diffusion, named after Martin Knudsen, is a means of diffusion that occurs when the scale length of a system is comparable to or smaller than the mean free path of the particles involved. An example of this is in a long pore with a narrow diameter (2–50 nm) because molecules frequently collide with the pore wall.
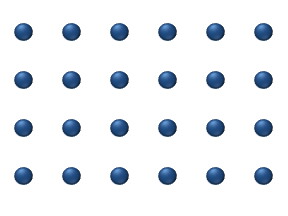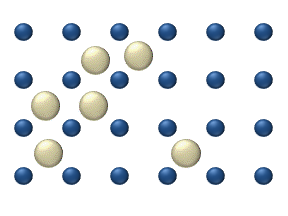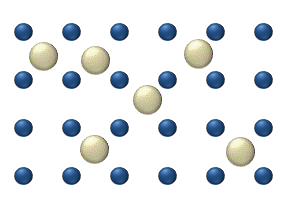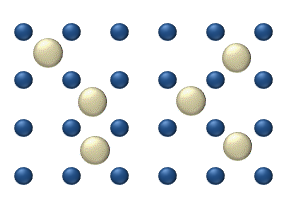 W
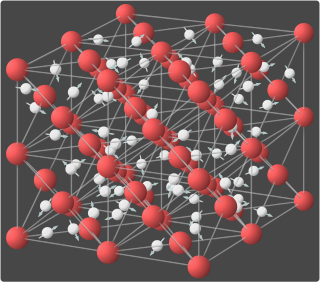
WLattice diffusion refers to atomic diffusion within a crystalline lattice. Diffusion within the crystal lattice occurs by either interstitial or substitutional mechanisms and is referred to as lattice diffusion. In interstitial lattice diffusion, a diffusant, will diffuse in between the lattice structure of another crystalline element. In substitutional lattice diffusion, the atom can only move by substituting place with another atom. Substitutional lattice diffusion is often contingent upon the availability of point vacancies throughout the crystal lattice. Diffusing particles migrate from point vacancy to point vacancy by the rapid, essentially random jumping about. Since the prevalence of point vacancies increases in accordance with the Arrhenius equation, the rate of crystal solid state diffusion increases with temperature. For a single atom in a defect-free crystal, the movement can be described by the "random walk" model.
 W
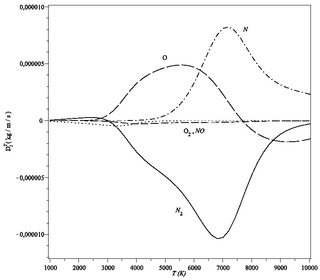
WLiesegang rings are a phenomenon seen in many, if not most, chemical systems undergoing a precipitation reaction under certain conditions of concentration and in the absence of convection. Rings are formed when weakly soluble salts are produced from reaction of two soluble substances, one of which is dissolved in a gel medium. The phenomenon is most commonly seen as rings in a Petri dish or bands in a test tube; however, more complex patterns have been observed, such as dislocations of the ring structure in a Petri dish, helices, and "Saturn rings" in a test tube. Despite continuous investigation since rediscovery of the rings in 1896, the mechanism for the formation of Liesegang rings is still unclear.
 W
WThe Maxwell–Stefan diffusion is a model for describing diffusion in multicomponent systems. The equations that describe these transport processes have been developed independently and in parallel by James Clerk Maxwell for dilute gases and Josef Stefan for fluids. The Maxwell–Stefan equation is∇: vector differential operator χ: Mole fraction μ: Chemical potential a: Activity i, j: Indexes for component i and j n: Number of components : Maxwell–Stefan-diffusion coefficient : Diffusion velocity of component i : Molar concentration of component i c: Total molar concentration : Flux of component i
 W
WMolecular diffusion, often simply called diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size (mass) of the particles. Diffusion explains the net flux of molecules from a region of higher concentration to one of lower concentration. Once the concentrations are equal the molecules continue to move, but since there is no concentration gradient the process of molecular diffusion has ceased and is instead governed by the process of self-diffusion, originating from the random motion of the molecules. The result of diffusion is a gradual mixing of material such that the distribution of molecules is uniform. Since the molecules are still in motion, but an equilibrium has been established, the end result of molecular diffusion is called a "dynamic equilibrium". In a phase with uniform temperature, absent external net forces acting on the particles, the diffusion process will eventually result in complete mixing.
 W
WOsmosis is the spontaneous net movement of solvent molecules through a selectively permeable membrane into a region of higher solute concentration, in the direction that tends to equalize the solute concentrations on the two sides. It may also be used to describe a physical process in which any solvent moves across a selectively permeable membrane separating two solutions of different concentrations. Osmosis can be made to do work. Osmotic pressure is defined as the external pressure required to be applied so that there is no net movement of solvent across the membrane. Osmotic pressure is a colligative property, meaning that the osmotic pressure depends on the molar concentration of the solute but not on its identity.
 W
WSemipermeable membrane is a type of biological or synthetic, polymeric membrane that will allow certain molecules or ions to pass through it by osmosis—or occasionally by more specialized processes of facilitated diffusion, passive transport or active transport. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials thicker than a membrane are also semipermeable. One example of this is the thin film on the inside of the egg. Note that a semipermeable membrane is not the same as a selectively permeable membrane. Semipermeable membrane describes a membrane that allows some particles to pass through, whereas the selectively permeable membrane will choose what passes through.
 W
WIn chemical engineering, a Stefan tube is a device that was devised by Josef Stefan in 1874. It is often used for measuring diffusion coefficients. It comprises a vertical tube, over the top of which a gas flows and at the bottom of which is a pool of volatile liquid that is maintained in a constant-temperature bath. The liquid in the pool evaporates, diffuses through the gas above it in the tube, and is carried away by the gas flow over the tube mouth at the top. One then measures the fall in the level of the liquid in the tube.
 W
WThe technology adoption lifecycle is a sociological model that describes the adoption or acceptance of a new product or innovation, according to the demographic and psychological characteristics of defined adopter groups. The process of adoption over time is typically illustrated as a classical normal distribution or "bell curve". The model indicates that the first group of people to use a new product is called "innovators", followed by "early adopters". Next come the early majority and late majority, and the last group to eventually adopt a product are called "Laggards" or "phobics." For example, a phobic may only use a cloud service when it is the only remaining method of performing a required task, but the phobic may not have an in-depth technical knowledge of how to use the service.
 W
WThe technology life-cycle (TLC) describes the commercial gain of a product through the expense of research and development phase, and the financial return during its "vital life". Some technologies, such as steel, paper or cement manufacturing, have a long lifespan while in other cases, such as electronic or pharmaceutical products, the lifespan may be quite short.