 W
WElectrochemistry is the branch of physical chemistry that studies the relationship between electricity, as a measurable and quantitative phenomenon, and identifiable chemical change, with either electricity considered an outcome of a particular chemical change or vice versa. These reactions involve electric charges moving between electrodes and an electrolyte. Thus electrochemistry deals with the interaction between electrical energy and chemical change.
 W
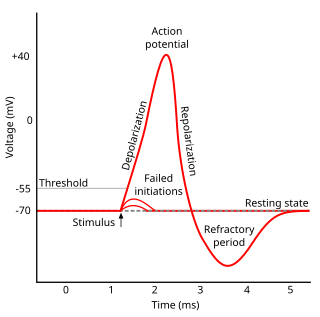
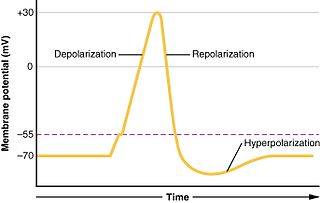
WIn physiology, an action potential (AP) occurs when the membrane potential of a specific cell location rapidly rises and falls: this depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of animal cells, called excitable cells, which include neurons, muscle cells, endocrine cells, glomus cells, and in some plant cells.
 W
WAfterhyperpolarization, or AHP, is the hyperpolarizing phase of a neuron's action potential where the cell's membrane potential falls below the normal resting potential. This is also commonly referred to as an action potential's undershoot phase. AHPs have been segregated into "fast", "medium", and "slow" components that appear to have distinct ionic mechanisms and durations. While fast and medium AHPs can be generated by single action potentials, slow AHPs generally develop only during trains of multiple action potentials.
 W
WBipolar electrochemistry is a phenomenon in electrochemistry based on the polarization of conducting objects in electric fields. Indeed, this polarization generates a potential difference between the two extremities of the substrate that is equal to the electric field value multiplied by the size of the object. If this potential difference is important enough, then redox reactions can be generated at the extremities of the object, oxidations will occur at one extremity coupled simultaneously to reductions at the other extremity. In a simple experimental setup consisting of a platinum wire in a weighing boat containing a pH indicator solution, a 30 V voltage across two electrodes will cause water reduction at one end of the wire and a pH increase and water oxidation at the anodic end and a pH decrease. The poles of the bipolar electrode also align themselves with the applied electric field.
 W
WA counterion is the ion that accompanies an ionic species in order to maintain electric neutrality. In table salt (NaCl), the sodium ion is the counterion for the chlorine ion and vice versa.
 W
WIn biology, depolarization is a change within a cell, during which the cell undergoes a shift in electric charge distribution, resulting in less negative charge inside the cell. Depolarization is essential to the function of many cells, communication between cells, and the overall physiology of an organism.
 W
WDielectric spectroscopy measures the dielectric properties of a medium as a function of frequency. It is based on the interaction of an external field with the electric dipole moment of the sample, often expressed by permittivity.
 W
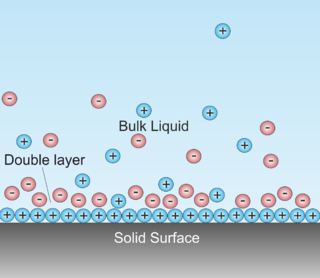
WA double layer is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge, consists of ions adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer".
 W
WA dry cell is a type of electric battery, commonly used for portable electrical devices. It was developed in 1886 by the German scientist Carl Gassner, after development of wet zinc-carbon batteries by Georges Leclanché in 1866. The modern version was developed by Japanese Yai Sakizo in 1887.
 W
WAn electrical conductivity meter measures the electrical conductivity in a solution. It has multiple applications in research and engineering, with common usage in hydroponics, aquaculture, aquaponics, and freshwater systems to monitor the amount of nutrients, salts or impurities in the water.
 W
WElectroosmotic flow is the motion of liquid induced by an applied potential across a porous material, capillary tube, membrane, microchannel, or any other fluid conduit. Because electroosmotic velocities are independent of conduit size, as long as the electrical double layer is much smaller than the characteristic length scale of the channel, electroosmotic flow will have little effect. Electroosmotic flow is most significant when in small channels. Electroosmotic flow is an essential component in chemical separation techniques, notably capillary electrophoresis. Electroosmotic flow can occur in natural unfiltered water, as well as buffered solutions.
 W
WAn electrocatalyst is a catalyst that participates in electrochemical reactions. Catalyst materials modify and increase the rate of chemical reactions without being consumed in the process. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or may be the electrode surface itself. An electrocatalyst can be heterogeneous such as a platinum surface or nanoparticles, or homogeneous like a coordination complex or enzyme. The electrocatalyst assists in transferring electrons between the electrode and reactants, and/or facilitates an intermediate chemical transformation described by an overall half-reaction.
 W
WAn Electrochemical aptamer-based (E-AB) biosensor has the ability to generate an electrochemical signal in response to specific target binding in vivo The signal is measured by a change in Faradaic current passed through an electrode. E-AB sensors are advantageous over previously reported aptamer-based sensors, such as fluorescence generating aptamers, due to their ability to detect target binding in vivo with real-time measurements. An E-AB sensor is composed of a three-electrode cell: an interrogating electrode, a reference electrode, and a counter electrode. A signal is generated within the electrochemical cell then measured and analyzed by a potentiostat. There are several biochemical and electrochemical parameters to optimize signal gain for E-AB biosensors. The density packing of DNA or RNA aptamers, the ACV frequency administered by the potentiostat, and the chemistry of the SAM are all factors that determine signal gain as well as the signal to noise ratio of target binding. E-AB biosensors provide a promising mechanism for in-situ sensing and feedback-controlled drug administration.
 W
WAn electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consists of two parts, the chemical gradient, or difference in solute concentration across a membrane, and the electrical gradient, or difference in charge across a membrane. When there are unequal concentrations of an ion across a permeable membrane, the ion will move across the membrane from the area of higher concentration to the area of lower concentration through simple diffusion. Ions also carry an electric charge that forms an electric potential across a membrane. If there is an unequal distribution of charges across the membrane, then the difference in electric potential generates a force that drives ion diffusion until the charges are balanced on both sides of the membrane.
 W
WElectrochemical quartz crystal microbalance (EQCM) is the combination of electrochemistry and quartz crystal microbalance, which was generated in the eighties. Typically, an EQCM device contains an electrochemical cells part and a QCM part. Two electrodes on both sides of the quartz crystal serve two purposes. Firstly, an alternating electric field is generated between the two electrodes for making up the oscillator. Secondly, the electrode contacting electrolyte is used as a working electrode (WE), together with a counter electrode (CE) and a reference electrode (RE), in the potentiostatic circuit constituting the electrochemistry cell. Thus, the working electrode of electrochemistry cell is the sensor of QCM.
 W
WThe Electrochemical Society is a learned society based in the United States that supports scientific inquiry in the field of electrochemistry and solid-state science and technology. The society membership comprises more than 8,000 scientists and engineers in over 70 countries worldwide who hold individual membership, as well as roughly 100 corporations and laboratories that hold corporate membership.
 W
WIn chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential.
 W
WThe absorption of electromagnetic radiation by water depends on the state of the water.
 W
WFaraday's laws of electrolysis are quantitative relationships based on the electrochemical research published by Michael Faraday in 1833.
 W
WIn materials science, fast ion conductors are solids with highly mobile ions. These materials are important in the area of solid-state ionics, and are also known as solid electrolytes and superionic conductors. These materials are useful in batteries and various sensors. Fast ion conductors are used primarily in solid oxide fuel cells. As solid electrolytes they allow the movement of ions without the need for a liquid or soft membrane separating the electrodes. The phenomenon relies on the hopping of ions through an otherwise rigid crystal structure.
 W
WFLiNaK is the name of the ternary eutectic alkaline metal fluoride salt mixture LiF-NaF-KF (46.5-11.5-42 mol %). It has a melting point of 454 °C and a boiling point of 1570 °C. It is used as electrolyte for the electroplating of refractory metals and compounds like titanium, tantalum, hafnium, zirconium and their borides. FLiNaK also could see potential use as a coolant in the very high temperature reactor, a type of nuclear reactor.
 W
WA flow battery, or redox flow battery, is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids that are pumped through the system on separate sides of a membrane. Ion exchange occurs through the membrane while both liquids circulate in their own respective space. Cell voltage is chemically determined by the Nernst equation and ranges, in practical applications, from 1.0 to 2.43 volts.
 W
WA Frost diagram or Frost–Ebsworth diagram is a type of graph used by inorganic chemists in electrochemistry to illustrate the relative stability of a number of different oxidation states of a particular substance. The graph illustrates the free energy vs oxidation state of a chemical species. This effect is dependent on pH, so this parameter also must be included. The free energy is determined by the oxidation–reduction half-reactions. The Frost diagram allows easier comprehension of these reduction potentials than the earlier-designed Latimer diagram, because the “lack of additivity of potentials” was confusing. The free energy ΔG° is related to reduction potential E in the graph by given formula: ΔG° = −nFE° or nE° = −ΔG°/F, where n is the number of transferred electrons, and F is Faraday constant. The Frost diagram is named after Arthur Atwater Frost, who originally created it as a way to "show both free energy and oxidation potential data conveniently" in a 1951 paper.
 W
WA galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection (CP) system used to protect buried or submerged metal structures from corrosion.
 W
WGalvanism is a term invented by the late 18th C. physicist and chemist, Alessandro Volta, to refer to the generation of electrical current by chemical action. The term also came to refer to the discoveries of its namesake, Luigi Galvani, specifically the generation of electrical current within biological organisms and the contraction/convulsion of biological muscle tissue upon contact with electrical current. While Volta theorized and later demonstrated the phenomenon of his "Galvanism" to be replicable with otherwise inert materials, Galvani thought his discovery to be a confirmation of the existence of "animal electricity," a vital force which gave life to organic matter.
 W
WNorman Hackerman was an American chemist, professor, and academic administrator who served as the 18th President of the University of Texas at Austin (1967–1970) and later as the 4th President of Rice University (1970–1985). He was an internationally known expert in metal corrosion.
 W
WInduced-charge electrokinetics in physics is the electrically driven fluid flow and particle motion in a liquid electrolyte. Consider a metal particle in contact with an aqueous solution in a chamber/channel. If different voltages apply to the end of this chamber/channel, electric field will generate in this chamber/channel. This applied electric field passes through this metal particle and causes the free charges inside the particle migrate under the skin of particle. As a result of this migration, the negative charges moves to the side which is close to the positive voltage while the positive charges moves to the opposite side of the particle. These charges under the skin of conducting particle attract the counter-ions of the aqueous solution; thus, the electric double layer (EDL) forms around the particle. The EDL sing on the surface of the conducting particle changes from positive to negative and the distribution of the charges varies along the particle geometry. Due to these variations, the EDL is non-uniform and has different sings. Thus, the induced zeta potential around the particle, and consequently slip velocity on the surface of the particle, vary as a function of local electric field. Differences in magnitude and direction of slip velocity on the surface of the conducting particle effects the flow pattern around this particle and causes micro vortices. Yasaman Daghighi and Dongqing Li, for the first time, experimentally illustrated these induced vortices around a 1.2mm diameter carbon-steel sphere under the 40V/cm direct current (DC) external electric filed. Chenhui Peng et al. also experimentally showed the patterns of electro-osmotic flow around an Au sphere when alternating current (AC) is involved . Electrokinetics here refers to a branch of science related to the motion and reaction of charged particles to the applied electric filed and its effects on its environment. It is sometimes referred as non-linear electrokinetic phenomena as well.
 W
WINT is a commonly used tetrazolium salt, similar to tetrazolium chloride that on reduction produces a red formazan dye that can be used for quantitative redox assays. It is also toxic to prokaryotes.
 W
WIonic conductivity is a measure of a substance's tendency towards ionic conduction. This involves the movement of an ion from one site to another through defects in the crystal lattice of a solid or aqueous solution.
 W
WSimilar to Pourbaix's diagram for the speciation of redox species as a function of the redox potential and the pH, ionic partition diagrams indicate in which an acid or a base are predominantly present in a biphasic system as a function of the Galvani potential difference between the two phases and the pH of the aqueous solution
 W
WAn ion-sensitive field-effect transistor (ISFET) is a field-effect transistor used for measuring ion concentrations in solution; when the ion concentration (such as H+, see pH scale) changes, the current through the transistor will change accordingly. Here, the solution is used as the gate electrode. A voltage between substrate and oxide surfaces arises due to an ion sheath. It is a special type of MOSFET (metal-oxide-semiconductor field-effect transistor), and shares the same basic structure, but with the metal gate replaced by an ion-sensitive membrane, electrolyte solution and reference electrode. Invented in 1970, the ISFET was the first biosensor FET (BioFET).
 W
WAn ion-sensitive field-effect transistor (ISFET) is a field-effect transistor used for measuring ion concentrations in solution; when the ion concentration (such as H+, see pH scale) changes, the current through the transistor will change accordingly. Here, the solution is used as the gate electrode. A voltage between substrate and oxide surfaces arises due to an ion sheath. It is a special type of MOSFET (metal-oxide-semiconductor field-effect transistor), and shares the same basic structure, but with the metal gate replaced by an ion-sensitive membrane, electrolyte solution and reference electrode. Invented in 1970, the ISFET was the first biosensor FET (BioFET).
 W
WAn ion-sensitive field-effect transistor (ISFET) is a field-effect transistor used for measuring ion concentrations in solution; when the ion concentration (such as H+, see pH scale) changes, the current through the transistor will change accordingly. Here, the solution is used as the gate electrode. A voltage between substrate and oxide surfaces arises due to an ion sheath. It is a special type of MOSFET (metal-oxide-semiconductor field-effect transistor), and shares the same basic structure, but with the metal gate replaced by an ion-sensitive membrane, electrolyte solution and reference electrode. Invented in 1970, the ISFET was the first biosensor FET (BioFET).
 W
WA liquid rheostat or water rheostat or salt water rheostat is a type of variable resistor. This may be used as a dummy load or as a starting resistor for large slip ring motors.
 W
WThe Marchywka effect refers to electrochemical cleaning of diamond using an electric field induced with remote electrodes.
 W
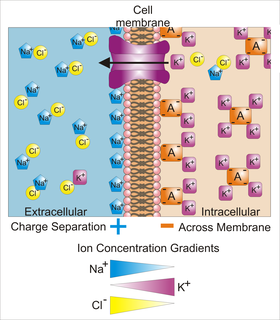
WMembrane potential is the difference in electric potential between the interior and the exterior of a biological cell. For the exterior of the cell, typical values of membrane potential, normally given in units of millivolts and denoted as mV, range from –40 mV to –80 mV.
 W
WMixed conductors, also known as mixed ion-electron conductors (MIEC), are a single-phase material that has significant conduction ionically and electronically. Due to the mixed conduction, a formally neutral species can transport in a solid and therefore mass storage and redistribution are enabled. Mixed conductors are well known in conjugation with high-temperature superconductivity and are able to capacitate rapid solid-state reactions.
 W
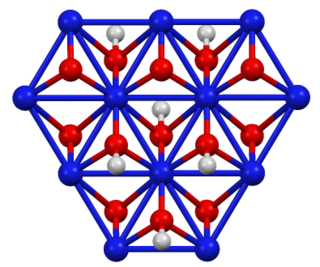
WNASICON is an acronym for sodium (Na) Super Ionic CONductor, which usually refers to a family of solids with the chemical formula Na1+xZr2SixP3−xO12, 0 < x < 3. In a broader sense, it is also used for similar compounds where Na, Zr and/or Si are replaced by isovalent elements. NASICON compounds have high ionic conductivities, on the order of 10−3 S/cm, which rival those of liquid electrolytes. They are caused by hopping of Na ions among interstitial sites of the NASICON crystal lattice.
 W
WNickel oxide hydroxide is the inorganic compound with the chemical formula NiO(OH). It is a black solid that is insoluble in all solvents but attacked by base and acid. It is a component of the nickel-metal hydride battery and of the nickel–iron battery.
 W
WNickel(III) oxide is the inorganic compound with the formula Ni2O3. It is not well characterized, and is sometimes referred to as black nickel oxide. Traces of Ni2O3 on nickel surfaces have been mentioned.
 W
WIn chemistry, an oxidizing agent, or oxidising agent (oxidiser) is a substance that has the ability to oxidize other substances — in other words to accept their electrons. Common oxidizing agents are oxygen, hydrogen peroxide and the halogens.
 W
WPaschen's law is an equation that gives the breakdown voltage, that is, the voltage necessary to start a discharge or electric arc, between two electrodes in a gas as a function of pressure and gap length. It is named after Friedrich Paschen who discovered it empirically in 1889.
 W
WA pH meter is a scientific instrument that measures the hydrogen-ion activity in water-based solutions, indicating its acidity or alkalinity expressed as pH. The pH meter measures the difference in electrical potential between a pH electrode and a reference electrode, and so the pH meter is sometimes referred to as a "potentiometric pH meter". The difference in electrical potential relates to the acidity or pH of the solution. The pH meter is used in many applications ranging from laboratory experimentation to quality control.
 W
WThe photoelectric effect is the emission of electrons when electromagnetic radiation, such as light, hits a material. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physics, and solid state and quantum chemistry to draw inferences about the properties of atoms, molecules and solids. The effect has found use in electronic devices specialized for light detection and precisely timed electron emission.
 W
WPhotovoltaics (PV) is the conversion of light into electricity using semiconducting materials that exhibit the photovoltaic effect, a phenomenon studied in physics, photochemistry, and electrochemistry. The photovoltaic effect is commercially utilized for electricity generation and as photosensors.
 W
WIn electrochemistry, and more generally in solution chemistry, a Pourbaix diagram, also known as a potential/pH diagram, EH–pH diagram or a pE/pH diagram, is a plot of possible thermodynamically stable phases of an aqueous electrochemical system. Boundaries (50 %/50 %) between the predominant chemical species are represented by lines. As such a Pourbaix diagram can be read much like a standard phase diagram with a different set of axes. Similarly to phase diagrams, they do not allow for reaction rate or kinetic effects. Beside potential and pH, the equilibrium concentrations are also dependent upon, e.g., temperature, pressure, and concentration. Pourbaix diagrams are commonly given at room temperature, atmospheric pressure, and molar concentrations of 10−6 and changing any of these parameters will yield a different diagram.
 W
WA proton conductor is an electrolyte, typically a solid electrolyte, in which H+ are the primary charge carriers.
 W
WPulse electrolysis is an alternate electrolysis method that utilises a pulsed direct current to initiate non-spontaneous chemical reactions. Also known as pulsed direct current (PDC) electrolysis, the increased number of variables that it introduces to the electrolysis method can change the application of the current to the electrodes and the resulting outcome. This varies from direct current (DC) electrolysis, which only allows the variation of one value, the voltage applied. By utilising conventional pulse width modulation (PMW), multiple dependent variables can be altered, including the type of waveform, typically a rectangular pulse wave, and the duty cycle, which determines the waveform frequency.
 W
WThe Pulvermacher chain, or in full as it was sold the Pulvermacher hydro-electric chain, was a type of voltaic battery sold in the second half of the 19th century for medical applications. Its chief market was amongst the numerous quack practitioners who were taking advantage of the popularity of the relatively new treatment of electrotherapy, or "electrification" as it was then known. Its unique selling point was its construction of numerous linked cells, rendering it mechanically flexible. A variant intended to be worn wrapped on part of the body for long periods was known as Pulvermacher's galvanic chain or electric belt.
 W
WQuantum photoelectrochemistry is the investigation of the quantum mechanical nature of photoelectrochemistry, the subfield of study within physical chemistry concerned with the interaction of light with electrochemical systems, typically through the application of quantum chemical calculations. Quantum photoelectrochemistry provides an expansion of quantum electrochemistry to processes involving also the interaction with light (photons). It therefore also includes essential elements of photochemistry. Key aspects of quantum photoelectrochemistry are calculations of optical excitations, photoinduced electron and energy transfer processes, excited state evolution, as well as interfacial charge separation and charge transport in nanoscale energy conversion systems.
 W
WIn neuroscience, repolarization refers to the change in membrane potential that returns it to a negative value just after the depolarization phase of an action potential which has changed the membrane potential to a positive value. The repolarization phase usually returns the membrane potential back to the resting membrane potential. The efflux of potassium (K+) ions results in the falling phase of an action potential. The ions pass through the selectivity filter of the K+ channel pore.
 W
WA salt bridge or ion bridge, in electrochemistry, is a laboratory device used to connect the oxidation and reduction half-cells of a galvanic cell, a type of electrochemical cell. It maintains electrical neutrality within the internal circuit. If no salt bridge were present, the solution in one half cell would accumulate negative charge and the solution in the other half cell would accumulate positive charge as the reaction proceeded, quickly preventing further reaction, and hence production of electricity.
 W
WSolar energy conversion describes technologies devoted to the transformation of solar energy to other (useful) forms of energy, including electricity, fuel, and heat. It covers light-harvesting technologies including traditional semiconductor photovoltaic devices (PVs), emerging photovoltaics, solar fuel generation via electrolysis, artificial photosynthesis, and related forms of photocatalysis directed at the generation of energy rich molecules.
 W
WSolid-state ionics is the study of ionic-electronic mixed conductor and fully ionic conductors and their uses. Some materials that fall into this category include inorganic crystalline and polycrystalline solids, ceramics, glasses, polymers, and composites. Solid-state ionic devices, such as solid oxide fuel cells, can be much more reliable and long-lasting, especially under harsh conditions, than comparable devices with fluid electrolytes.
 W
WSpectroelectrochemistry (SEC) is a set of multi-response analytical techniques in which complementary chemical information is obtained in a single experiment. Spectroelectrochemistry provides a whole vision of the phenomena that take place in the electrode process. The first spectroelectrochemical experiment was carried out by Kuwana in 1964.
 W
WA thermogalvanic cell is a kind of galvanic cell in which heat is employed to provide electrical power directly. These cells are electrochemical cells in which the two electrodes are deliberately maintained at different temperatures. This temperature difference generates a potential difference between the electrodes. The electrodes can be of identical composition and the electrolyte solution homogeneous. This is usually the case in these cells. This is in contrast to galvanic cells in which electrodes and/or solutions of different composition provide the electromotive potential. As long as there is a difference in temperature between the electrodes a current will flow through the circuit. A thermogalvanic cell can be seen as analogous to a concentration cell but instead of running on differences in the concentration/pressure of the reactants they make use of differences in the "concentrations" of thermal energy. The principal application of thermogalvanic cells is the production of electricity from low-temperature heat sources. Their energetic efficiency is low, in the range of 0.1% to 1% for conversion of heat into electricity.
 W
WYttria-stabilized zirconia (YSZ) is a ceramic in which the cubic crystal structure of zirconium dioxide is made stable at room temperature by an addition of yttrium oxide. These oxides are commonly called "zirconia" (ZrO2) and "yttria" (Y2O3), hence the name.
 W
W'Jose H. Zagal Moya is a Chilean scientist educated at the University of Chile with postgraduate training in the United States of America with a Ph.D.degree from Case Western Reserve University, Cleveland Ohio and postdoctoral training at Brookhaven National Laboratory, Upton, New York. At present he is a Distinguished Professor, Department of Chemistry and Materials,Faculty of Chemistry and Biology, Universidad de Santiago de Chile (USACH) where he directs the Laboratory of Electrocatalysis since 1982. He got his Ph.D. in chemistry Case Western Reserve University, US (1978) and was postdoctoral fellow at Brookhaven National Laboratory, Upton, New York, in 1982. His main research efforts are focused on the fundamentals of electron transfer reactions that are relevant for energy conversion and sensors. He has contributed in the area of electrocatalysis, electrodes modified with metal macrocyclics, electrochemistry of biological molecules, the catalysis of the reduction of molecular oxygen and many other reactions of relevance, conductive polymers, electrochemical sensors and in pioneering work in the establishment of non-linear correlations between thermodynamic properties of molecular catalysts and their electrochemical reactivity. These contributions are essential in the development of non-precious metal catalysts for energy conversion devices and electrochemical sensors. [1][2][3] He also has contributed in the field of corrosion, conductive polymers and his well-known volcano correlations for the electrocatalytic properties of surface-confined molecular catalysts
 W
W