 W
WThe emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an atom or molecule making a transition from a high energy state to a lower energy state. The photon energy of the emitted photon is equal to the energy difference between the two states. There are many possible electron transitions for each atom, and each transition has a specific energy difference. This collection of different transitions, leading to different radiated wavelengths, make up an emission spectrum. Each element's emission spectrum is unique. Therefore, spectroscopy can be used to identify elements in matter of unknown composition. Similarly, the emission spectra of molecules can be used in chemical analysis of substances.
 W
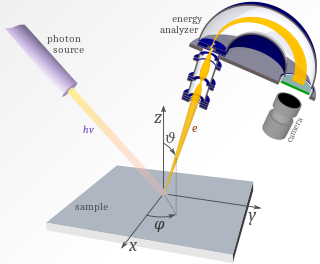
WAngle-resolved photoemission spectroscopy (ARPES) is an experimental technique used in condensed matter physics to probe the allowed energies and momenta of the electrons in a material, usually a crystalline solid. It is based on the photoelectric effect, in which an incoming photon of sufficient energy ejects an electron from the surface of a material. By directly measuring the kinetic energy and emission angle distributions of the emitted photoelectrons, the technique can map the electronic band structure and Fermi surfaces. ARPES is best suited for the study of one- or two-dimensional materials. It has been used by physicists to investigate high-temperature superconductors, graphene, topological materials, quantum well states, and materials exhibiting charge density waves.
 W
WAtomic emission spectroscopy (AES) is a method of chemical analysis that uses the intensity of light emitted from a flame, plasma, arc, or spark at a particular wavelength to determine the quantity of an element in a sample. The wavelength of the atomic spectral line in the emission spectrum gives the identity of the element while the intensity of the emitted light is proportional to the number of atoms of the element. The sample may be excited by various methods.
 W
WChemiluminescence is the emission of light (luminescence) as the result of a chemical reaction. There may also be limited emission of heat. Given reactants A and B, with an excited intermediate ◊,[A] + [B] → [◊] → [Products] + light
 W
WIn astronomy and in astrophysics, for radiative losses of the solar corona, it is meant the energy flux radiated from the external atmosphere of the Sun, and, in particular, the processes of production of the radiation coming from the solar corona and transition region, where the plasma is optically-thin. On the contrary, in the chromosphere, where the temperature decreases from the photospheric value of 6000 K to the minimum of 4400 K, the optical depth is about 1, and the radiation is thermal.
 W
WEinstein coefficients are mathematical quantities which are a measure of the probability of absorption or emission of light by an atom or molecule. The Einstein A coefficients are related to the rate of spontaneous emission of light, and the Einstein B coefficients are related to the absorption and stimulated emission of light.
 W
WThe emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an atom or molecule making a transition from a high energy state to a lower energy state. The photon energy of the emitted photon is equal to the energy difference between the two states. There are many possible electron transitions for each atom, and each transition has a specific energy difference. This collection of different transitions, leading to different radiated wavelengths, make up an emission spectrum. Each element's emission spectrum is unique. Therefore, spectroscopy can be used to identify elements in matter of unknown composition. Similarly, the emission spectra of molecules can be used in chemical analysis of substances.
 W
WThe equivalent width of a spectral line is a measure of the area of the line on a plot of intensity versus wavelength. It is found by forming a rectangle with a height equal to that of continuum emission, and finding the width such that the area of the rectangle is equal to the area in the spectral line. It is a measure of the strength of spectral features that is primarily used in astronomy.
 W
WGlow-discharge optical emission spectroscopy (GDOES) is a spectroscopic method for the quantitative analysis of metals and other non-metallic solids. The idea was published and patented in 1968 by Werner Grimm from Hanau, Germany.
 W
WThe hydrogen line, 21-centimeter line, or H I line is the electromagnetic radiation spectral line that is created by a change in the energy state of neutral hydrogen atoms. This electromagnetic radiation has a precise frequency of 1420405751.768(2) Hz, which is equivalent to the vacuum wavelength of 21.106114054160(30) cm in free space. This wavelength falls below the microwave region of the electromagnetic spectrum, which begins at 3.0 GHz, and it is observed frequently in radio astronomy because those radio waves can penetrate the large clouds of interstellar cosmic dust that are opaque to visible light. This line is also the theoretical basis of the hydrogen maser.
 W
WThe emission spectrum of atomic hydrogen has been divided into a number of spectral series, with wavelengths given by the Rydberg formula. These observed spectral lines are due to the electron making transitions between two energy levels in an atom. The classification of the series by the Rydberg formula was important in the development of quantum mechanics. The spectral series are important in astronomical spectroscopy for detecting the presence of hydrogen and calculating red shifts.
 W
WInductively coupled plasma atomic emission spectroscopy (ICP-AES), also referred to as inductively coupled plasma optical emission spectrometry (ICP-OES), is an analytical technique used for the detection of chemical elements. It is a type of emission spectroscopy that uses the inductively coupled plasma to produce excited atoms and ions that emit electromagnetic radiation at wavelengths characteristic of a particular element. The plasma is a high temperature source of ionised source gas. The plasma is sustained and maintained by inductive coupling from cooled electrical coils at megahertz frequencies. The source temperature is in the range from 6000 to 10,000 K. The intensity of the emissions from various wavelengths of light are proportional to the concentrations of the elements within the sample.
 W
WLaser-induced breakdown spectroscopy (LIBS) is a type of atomic emission spectroscopy which uses a highly energetic laser pulse as the excitation source. The laser is focused to form a plasma, which atomizes and excites samples. The formation of the plasma only begins when the focused laser achieves a certain threshold for optical breakdown, which generally depends on the environment and the target material.
 W
WLyman continuum photons, shortened to Ly continuum photons or Lyc photons, are the photons emitted from stars at photon energies above the Lyman limit. Hydrogen is ionized by absorbing LyC. Working from Victor Schumann's discovery of ultraviolet light, from 1906 to 1914, Theodore Lyman observed that atomic hydrogen absorbs light only at specific frequencies and the Lyman series is thus named after him. All the wavelengths in the Lyman series are in the ultraviolet band. This quantized absorption behavior occurs only up to an energy limit, known as the ionization energy. In the case of neutral atomic hydrogen, the minimum ionization energy is equal to the Lyman limit, where the photon has enough energy to completely ionize the atom, resulting in a free proton and a free electron. Above this energy, all wavelengths of light may be absorbed. This forms a continuum in the energy spectrum; the spectrum is continuous rather than composed of many discrete lines, which are seen at lower energies.
 W
WPhotoemission spectroscopy (PES), also known as photoelectron spectroscopy, refers to energy measurement of electrons emitted from solids, gases or liquids by the photoelectric effect, in order to determine the binding energies of electrons in the substance. The term refers to various techniques, depending on whether the ionization energy is provided by X-ray, XUV or UV photons. Regardless of the incident photon beam, however, all photoelectron spectroscopy revolves around the general theme of surface analysis by measuring the ejected electrons.
 W
WIn atomic emission spectroscopy, the principal series is a series of spectral lines caused when electrons move between p orbitals of an atom and the lowest available s orbital. These lines are usually found in the visible and ultraviolet portions of the electromagnetic spectrum. The principal series has given the letter p to the p atomic orbital and subshell.
 W
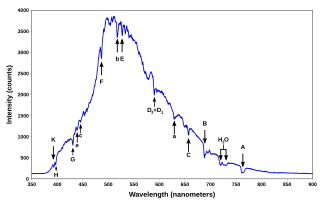
WA spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from emission or absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to identify atoms and molecules. These "fingerprints" can be compared to the previously collected ones of atoms and molecules, and are thus used to identify the atomic and molecular components of stars and planets, which would otherwise be impossible.
 W
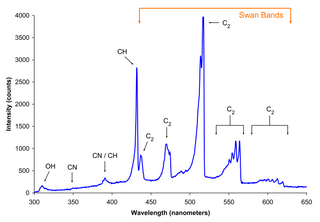
WSwan bands are a characteristic of the spectra of carbon stars, comets and of burning hydrocarbon fuels. They are named for the Scottish physicist William Swan, who first studied the spectral analysis of radical diatomic carbon (C2) in 1856.
 W
WTime-resolved two-photon photoelectron (2PPE) spectroscopy is a time-resolved spectroscopy technique which is used to study electronic structure and electronic excitations at surfaces. The technique utilizes femtosecond to picosecond laser pulses in order to first photoexcite an electron. After a time delay, the excited electron is photoemitted into a free electron state by a second pulse. The kinetic energy and the emission angle of the photoelectron are measured in an electron energy analyzer. To facilitate investigations on the population and relaxation pathways of the excitation, this measurement is performed at different time delays.
 W
WX-ray emission spectroscopy (XES) is a form of X-ray spectroscopy in which the X-ray line spectra are measured with a spectral resolution sufficient to analyze the impact of the chemical environment on the X-ray line energy and on branching ratios. This is done by exciting electrons out of their shell and then watching the emitted photons of the recombinating electrons.
 W
WX-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique based on the photoelectric effect that can identify the elements that exist within a material or are covering its surface, as well as their chemical state, and the overall electronic structure and density of the electronic states in the material. XPS is a powerful measurement technique because it not only shows what elements are present, but also what other elements they are bonded to. The technique can be used in line profiling of the elemental composition across the surface, or in depth profiling when paired with ion-beam etching. It is often applied to study chemical processes in the materials in their as-received state or after cleavage, scraping, exposure to heat, reactive gasses or solutions, ultraviolet light, or during ion implantation.