 W
WAlkalinity (from Arabic "al-qalī") is the capacity of water to resist acidification. It should not be confused with basicity which is an absolute measurement on the pH scale. Alkalinity is the strength of a buffer solution composed of weak acids and their conjugate bases. It is measured by titrating the solution with an acid such as HCl until its pH changes abruptly, or it reaches a known endpoint where that happens. Alkalinity is expressed in units of concentration, such as meq/L (milliequivalents per liter), μeq/kg (microequivalents per kilogram), or mg/L CaCO3 (milligrams per liter calcium carbonate). Each of these measurements corresponds to an amount of acid added as a titrant.
 W
WOceanic anoxic events or anoxic events (anoxia conditions) were intervals in the Earth's past where portions of oceans became depleted in oxygen (O2) over a large geographic areas. During some of these events, euxinia, waters that contain hydrogen sulfide, H2S, developed. Although anoxic events have not happened for millions of years, the geological record shows that they happened many times in the past. Anoxic events coincided with several mass extinctions and may have contributed to them. These mass extinctions include some that geobiologists use as time markers in biostratigraphic dating. Many geologists believe oceanic anoxic events are strongly linked to slowing of ocean circulation, climatic warming, and elevated levels of greenhouse gases. Researchers have proposed enhanced volcanism (the release of CO2) as the "central external trigger for euxinia".
 W
WThe Biogeography of Deep-Water Chemosynthetic Ecosystems is a field project of the Census of Marine Life programme (CoML). The main aim of ChEss is to determine the biogeography of deep-water chemosynthetic ecosystems at a global scale and to understand the processes driving these ecosystems. ChEss addresses the main questions of CoML on diversity, abundance and distribution of marine species, focusing on deep-water reducing environments such as hydrothermal vents, cold seeps, whale falls, sunken wood and areas of low oxygen that intersect with continental margins and seamounts.
 W
WThe biological pump, also known as the marine carbon pump, is, in its simplest form, the ocean's biologically driven sequestration of carbon from the atmosphere and land runoff to the ocean interior and seafloor sediments. It is the part of the oceanic carbon cycle responsible for the cycling of organic matter formed mainly by phytoplankton during photosynthesis (soft-tissue pump), as well as the cycling of calcium carbonate (CaCO3) formed into shells by certain organisms such as plankton and mollusks (carbonate pump).
 W
WA Bjerrum plot (named after Niels Bjerrum) is a graph of the concentrations of the different species of a polyprotic acid in a solution, as a function of pH, when the solution is at equilibrium. Due to the many orders of magnitude spanned by the concentrations, they are commonly plotted on a logarithmic scale. Sometimes the ratios of the concentrations are plotted rather than the actual concentrations. Occasionally H+ and OH− are also plotted.
 W
WBlue carbon refers to carbon dioxide removed from the atmosphere by the world's coastal ocean ecosystems, mostly mangroves, salt marshes, seagrasses and potentially macroalgae, through plant growth and the accumulation and burial of organic matter in the soil.
 W
WThe carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth. Carbon is the main component of biological compounds as well as a major component of many minerals such as limestone. Along with the nitrogen cycle and the water cycle, the carbon cycle comprises a sequence of events that are key to make Earth capable of sustaining life. It describes the movement of carbon as it is recycled and reused throughout the biosphere, as well as long-term processes of carbon sequestration to and release from carbon sinks.
 W
WClimate change mitigation consists of actions to limit the magnitude or rate of global warming and its related effects. This generally involves reductions in human emissions of greenhouse gases (GHGs).
 W
WThe cold blob in the North Atlantic describes a cold temperature anomaly of ocean surface waters, affecting the Atlantic Meridional Overturning Circulation (AMOC) which is part of the thermohaline circulation, possibly related to global warming-induced melting of the Greenland ice sheet.
 W
WColored dissolved organic matter (CDOM) is the optically measurable component of dissolved organic matter in water. Also known as chromophoric dissolved organic matter, yellow substance, and gelbstoff, CDOM occurs naturally in aquatic environments and is a complex mixture of many hundreds to thousands of individual, unique organic matter molecules, which are primarily leached from decaying detritus and organic matter. CDOM most strongly absorbs short wavelength light ranging from blue to ultraviolet, whereas pure water absorbs longer wavelength red light. Therefore, water with little or no CDOM, such as the open ocean, appears blue. Waters containing high amounts of CDOM can range from brown, as in many rivers, to yellow and yellow-brown in coastal waters. In general, CDOM concentrations are much higher in fresh waters and estuaries than in the open ocean, though concentrations are highly variable, as is the estimated contribution of CDOM to the total dissolved organic matter pool.
 W
WIn oceanic biogeochemistry, the continental shelf pump is proposed to operate in the shallow waters of the continental shelves, acting as a mechanism to transport carbon from surface waters to the interior of the adjacent deep ocean.
 W
WDead zones are hypoxic (low-oxygen) areas in the world's oceans and large lakes, which causes these bodies of water to fail to support the marine life living there. Historically, many of these sites were naturally occurring. However, in the 1970s, oceanographers began noting increased instances and expanses of dead zones. These occur near inhabited coastlines, where aquatic life is most concentrated.
 W
WIn oceanic biogeochemistry, the f-ratio is the fraction of total primary production fuelled by nitrate. The ratio was originally defined by Richard Eppley and Bruce Peterson in one of the first papers estimating global oceanic production. This fraction was originally believed significant because it appeared to directly relate to the sinking (export) flux of organic marine snow from the surface ocean by the biological pump. However, this interpretation relied on the assumption of a strong depth-partitioning of a parallel process, nitrification, that more recent measurements has questioned.
 W
WHypoxia refers to low oxygen conditions. Normally, 20.9% of the gas in the atmosphere is oxygen. The partial pressure of oxygen in the atmosphere is 20.9% of the total barometric pressure. In water, oxygen levels are much lower, approximately 7 ppm 0.0007% in good quality water, and fluctuate locally depending on the presence of photosynthetic organisms and relative distance to the surface.
 W
WJelly-falls are marine carbon cycling events whereby gelatinous zooplankton, primarily cnidarians, sink to the seafloor and enhance carbon and nitrogen fluxes via rapidly sinking particulate organic matter. These events provide nutrition to benthic megafauna and bacteria. Jelly-falls have been implicated as a major “gelatinous pathway” for the sequestration of labile biogenic carbon through the biological pump. These events are common in protected areas with high levels of primary production and water quality suitable to support cnidarian species. These areas include estuaries and several studies have been conducted in fjords of Norway.
 W
WThe lipid pump is the sequestration of carbon from the ocean's surface to deeper waters through the usage of lipids by overwintering vertically migratory zooplankton. This carbon enters the deep ocean through respiration and mortality of the zooplankton in question. This lipid pump also entails a lipid shunt, where other nutrients like nitrogen and phosphorus that are consumed in excess must be excreted back to the surface environment. This means that the carbon transported due to the lipid pump does not affect essential nutrients in the ocean surface. The contribution of the lipid pump to the sequestering of carbon in the deeper waters of the ocean can be substantial: the carbon transported below 1,000 metres (3,300 ft) by copepods of the genus Calanus in the Arctic Ocean almost equals that transported below the same depth annually by particulate organic carbon.
 W
WIn the deep ocean, marine snow is a continuous shower of mostly organic detritus falling from the upper layers of the water column. It is a significant means of exporting energy from the light-rich photic zone to the aphotic zone below which is referred to as the biological pump. Export production is the amount of organic matter produced in the ocean by primary production that is not recycled (remineralised) before it sinks into the aphotic zone. Because of the role of export production in the ocean's biological pump, it is typically measured in units of carbon .The term was first coined by the explorer William Beebe as he observed it from his bathysphere. As the origin of marine snow lies in activities within the productive photic zone, the prevalence of marine snow changes with seasonal fluctuations in photosynthetic activity and ocean currents. Marine snow can be an important food source for organisms living in the aphotic zone, particularly for organisms which live very deep in the water column.
 W
WOcean acidification is the ongoing decrease in the pH of the Earth's oceans, caused by the uptake of carbon dioxide (CO2) from the atmosphere. Seawater is slightly basic (meaning pH > 7), and ocean acidification involves a shift towards pH-neutral conditions rather than a transition to acidic conditions (pH < 7). An estimated 30–40% of the carbon dioxide from human activity released into the atmosphere dissolves into oceans, rivers and lakes. Some of it reacts with the water to form carbonic acid. Some of the resulting carbonic acid molecules dissociate into a bicarbonate ion and a hydrogen ion, thus increasing ocean acidity (H+ ion concentration). Between 1751 and 1996, surface ocean pH is estimated to have decreased from approximately 8.25 to 8.14, representing an increase of almost 30% in H+ ion concentration in the world's oceans. Earth System Models project that, by around 2008, ocean acidity exceeded historical analogues and, in combination with other ocean biogeochemical changes, could undermine the functioning of marine ecosystems and disrupt the provision of many goods and services associated with the ocean beginning as early as 2100.
 W
WOcean acidification threatens the Great Barrier Reef by reducing the viability and strength of coral reefs. The Great Barrier Reef, considered one of the seven natural wonders of the world and a biodiversity hotspot, is located in Australia. Similar to other coral reefs, it is experiencing degradation due to ocean acidification. Ocean acidification results from a rise in atmospheric carbon dioxide, which is taken up by the ocean. This process can increase sea surface temperature, decrease aragonite, and lower the pH of the ocean.
 W
WThe oceanic carbon cycle is composed of processes that exchange carbon between various pools within the ocean as well as between the atmosphere, Earth interior, and the seafloor. The carbon cycle is a result of many interacting forces across multiple time and space scales that circulates carbon around the planet, ensuring that carbon is available globally. The Oceanic carbon cycle is a central process to the global carbon cycle and contains both inorganic carbon and organic carbon. Part of the marine carbon cycle transforms carbon between non-living and living matter.
 W
WThe oxygen cycle is the biogeochemical transitions of oxygen atoms between different oxidation states in ions, oxides, and molecules through redox reactions within and between the spheres/reservoirs of the planet Earth. The word oxygen in the literature typically refers to the most common oxygen allotrope, elemental/diatomic oxygen (O2), as it is a common product or reactant of many biogeochemical redox reactions within the cycle. Processes within the oxygen cycle are considered to be biological or geological and are evaluated as either a source (O2 production) or sink (O2 consumption).
 W
WThe oxygen minimum zone (OMZ), sometimes referred to as the shadow zone, is the zone in which oxygen saturation in seawater in the ocean is at its lowest. This zone occurs at depths of about 200 to 1,500 m (660–4,920 ft), depending on local circumstances. OMZs are found worldwide, typically along the western coast of continents, in areas where an interplay of physical and biological processes concurrently lower the oxygen concentration and restrict the water from mixing with surrounding waters, creating a “pool” of water where oxygen concentrations fall from the normal range of 4–6 mg/l to below 2 mg/l.
 W
WParticulate organic matter (POM) is a fraction of total organic matter operationally defined as that which does not pass through a filter pore size that typically ranges in size from 0.22 and 0.7 micrometers. The fraction that does pass through the filter is called dissolved organic matter (DOM).
 W
WThe pCO2, PCO2, or is the partial pressure of carbon dioxide (CO2), often used in reference to blood but also used in oceanography to describe the partial pressure of CO2 in the ocean and in life support systems engineering and underwater diving to describe the partial pressure in a breathing gas. Usually, the arterial blood is the relevant context; the symbol for in arterial blood is . Measurement of in the systemic circulation indicates the effectiveness of ventilation at the lungs' alveoli, given the diffusing capacity of the gas. It is a good indicator of respiratory function and the closely related factor of acid–base homeostasis, reflecting the amount of acid in the blood (without lactic acid).
 W
WThe National Science Foundation's (NSF) Ocean Observatories Initiative (OOI) Regional Scale Nodes (RSN) component is an electro-optically cabled underwater observatory that directly connects to the global Internet. It is the largest cable-linked seabed observatory in the world, and also the first of its kind in the United States.
 W
WSalinity is the saltiness or amount of salt dissolved in a body of water, called saline water. This is usually measured in . Salinity is an important factor in determining many aspects of the chemistry of natural waters and of biological processes within it, and is a thermodynamic state variable that, along with temperature and pressure, governs physical characteristics like the density and heat capacity of the water.
 W
WSea foam, ocean foam, beach foam, or spume is a type of foam created by the agitation of seawater, particularly when it contains higher concentrations of dissolved organic matter derived from sources such as the offshore breakdown of algal blooms. These compounds can act as surfactants or foaming agents. As the seawater is churned by breaking waves in the surf zone adjacent to the shore, the surfactants under these turbulent conditions trap air, forming persistent bubbles that stick to each other through surface tension. Sea foam is a global phenomenon and it varies depending on location and the potential influence of the surrounding marine, freshwater, and/or terrestrial environments. Due to its low density and persistence, foam can be blown by strong on-shore winds from the beach face inland.
 W
WSea salt is salt that is produced by the evaporation of seawater. It is used as a seasoning in foods, cooking, cosmetics and for preserving food. It is also called bay salt, solar salt, or salt. Like mined rock salt, production of sea salt has been dated to prehistoric times. There is no scientific evidence that consuming sea salt instead of more refined sodium chloride salts has any health benefit.
 W
WSeawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5%. This means that every kilogram of seawater has approximately 35 grams (1.2 oz) of dissolved salts. Average density at the surface is 1.025 kg/l. Seawater is denser than both fresh water and pure water because the dissolved salts increase the mass by a larger proportion than the volume. The freezing point of seawater decreases as salt concentration increases. At typical salinity, it freezes at about −2 °C (28 °F). The coldest seawater ever recorded was in 2010, in a stream under an Antarctic glacier, and measured −2.6 °C (27.3 °F). Seawater pH is typically limited to a range between 7.5 and 8.4. However, there is no universally accepted reference pH-scale for seawater and the difference between measurements based on different reference scales may be up to 0.14 units.
 W
WSel gris is a coarse granular sea salt popularized by the French. Sel gris comes from the same solar evaporation salt pans as fleur de sel but is harvested differently; it is allowed to come into contact with the bottom of the salt pan before being raked, hence its gray color. Sel gris is coarser than fleur de sel but is also a moist salt, typically containing 13 percent residual moisture.
 W
WIn oceanic biogeochemistry, the solubility pump is a physico-chemical process that transports carbon as dissolved inorganic carbon (DIC) from the ocean's surface to its interior.
 W
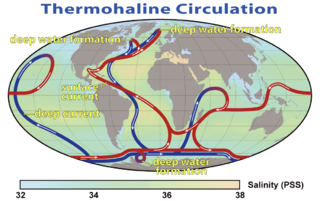
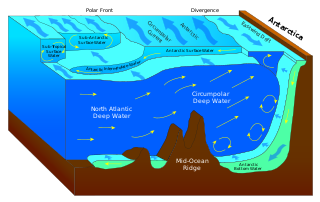
WThermohaline circulation (THC) is a part of the large-scale ocean circulation that is driven by global density gradients created by surface heat and freshwater fluxes. The adjective thermohaline derives from thermo- referring to temperature and -haline referring to salt content, factors which together determine the density of sea water. Wind-driven surface currents travel polewards from the equatorial Atlantic Ocean, cooling en route, and eventually sinking at high latitudes. This dense water then flows into the ocean basins. While the bulk of it upwells in the Southern Ocean, the oldest waters upwell in the North Pacific. Extensive mixing therefore takes place between the ocean basins, reducing differences between them and making the Earth's oceans a global system. The water in these circuits transport both energy and mass around the globe. As such, the state of the circulation has a large impact on the climate of the Earth.
 W
WDissolved inorganic carbon (DIC) is the sum of the aqueous species of inorganic carbon in a solution. Carbon compounds can be distinguished as either organic or inorganic, and as dissolved or particulate, depending on their composition. Organic carbon forms the backbone of key component of organic compounds such as – proteins, lipids, carbohydrates, and nucleic acids.
 W
WTotal inorganic carbon is the sum of inorganic carbon species.
 W
WVienna Standard Mean Ocean Water (VSMOW) is an isotopic standard for water. Despite the name, VSMOW is pure water with no salt or other chemicals found in the oceans. The VSMOW standard was promulgated by the International Atomic Energy Agency in 1968, and since 1993 continues to be evaluated and studied by the IAEA along with the European Institute for Reference Materials and Measurements and the American National Institute of Standards and Technology. The standard includes both the established values of stable isotopes found in waters and calibration materials provided for standardization and interlaboratory comparisons of instruments used to measure these values in experimental materials.
 W
WAn oceanographic water mass is an identifiable body of water with a common formation history which has physical properties distinct from surrounding water. Properties include temperature, salinity, chemical - isotopic ratios, and other physical quantities.